![]()
Page 2 Contents: #43 Ma, #72 Hf, #75 Re, #87 Fr, #95 Am, #97 Bk, #98 Cf, #105 Db, #108 Hs , #110 Ds

 Mendeleev´s periodic table guided searches for new elements. The difficulty separating rare earths, and the understanding of radioactive elements with differing half-lives was compounded because no one knew how many such elements there were, or how they should be incorporated into the periodic table. Henry Gwyn Jeffreys Moseley (1887-1915) found a method of using X-rays to assist the search for new elements. In 1914 he reported that the ray spectrum of an element is directly related to its atomic number. He had studied the spectra of rare earths and found unfilled spaces in the periodic table for elements #43, 61, 72, 75, 85, 87, and 91. When Great Britain entered World War I, Moseley left for the Dardanelles. On August 10, 1915 a Turkish bullet passed through his head before his 28th birthday.
Mendeleev´s periodic table guided searches for new elements. The difficulty separating rare earths, and the understanding of radioactive elements with differing half-lives was compounded because no one knew how many such elements there were, or how they should be incorporated into the periodic table. Henry Gwyn Jeffreys Moseley (1887-1915) found a method of using X-rays to assist the search for new elements. In 1914 he reported that the ray spectrum of an element is directly related to its atomic number. He had studied the spectra of rare earths and found unfilled spaces in the periodic table for elements #43, 61, 72, 75, 85, 87, and 91. When Great Britain entered World War I, Moseley left for the Dardanelles. On August 10, 1915 a Turkish bullet passed through his head before his 28th birthday.

 Niels Bohr had suggested that the outer
Niels Bohr had suggested that the outer valence
electrons determine chemical properties. Generally adjacent elements on the periodic chart have appreciable differences in chemical properties because of differing numbers of valence electrons. However, in the rare-earth group and the triads of the Iron and Platinum families, the electrons only differ at deeper levels, and therefore these elements are difficult to separate. Bohr used the new quantum theory to suggest the number of rare-earths. He predicted that element #72 should be quite different from the rare-earths between Lanthanium (#57) and Lutetium (#71), but should closely resemble Zirconium. Georg von Hevesy (left, from Hungary) and Dirk Coster (right, from the Netherlands), working at the Bohr Institute of Theoretical Physics in Copenhagen in 1923 found that Zirconium compounds do in fact contain 1 to 5% of element #72 which they called Hafnium (Hf = #72), (Latin) for Copenhagen. It was identified by Moseley´s method of x-ray spectroscopy after repeated recrystallization of double ammonium and potassium fluorides. This discovery required the atomic weight of Zirconium be revised downward because previous measurements included some Hafnium within the Zirconium.
 Walter Noddack,(1893-1960) Ida Eva Tacke,(1896-1978 pictured left and below right) and Otto Berg announced in 1925 the discovery of elements #43 and #75, the last missing elements on the main periodic table. Platinum ores were known to contain elements #24-29, 44-47, and 76-79, while columbite contains elements #39-42 and 72-74. So Noddack and Tacke at the Physico-Technical Testing Office in Berlin attempted to separate elements #43 and #75 from these materials with Berg at Werner-Siemens Laboratory doing the identification. The team found weak X-ray spectral lines when electrons excited the elements. Element #75 was separated from columbite and named Rhenium (Re), (Latin) for the Rhenany-Reinland of Ida Tacke´s birth. Tacke and Noddack married in 1926. Tacke went on to isolate a milligram of Rhenium in 1926 and determine its chemistry. It proved to be an expensive and difficult metal to work. Not produced again for a quarter century, 50 tons/year are now produced with 90% used as catalysts.
Walter Noddack,(1893-1960) Ida Eva Tacke,(1896-1978 pictured left and below right) and Otto Berg announced in 1925 the discovery of elements #43 and #75, the last missing elements on the main periodic table. Platinum ores were known to contain elements #24-29, 44-47, and 76-79, while columbite contains elements #39-42 and 72-74. So Noddack and Tacke at the Physico-Technical Testing Office in Berlin attempted to separate elements #43 and #75 from these materials with Berg at Werner-Siemens Laboratory doing the identification. The team found weak X-ray spectral lines when electrons excited the elements. Element #75 was separated from columbite and named Rhenium (Re), (Latin) for the Rhenany-Reinland of Ida Tacke´s birth. Tacke and Noddack married in 1926. Tacke went on to isolate a milligram of Rhenium in 1926 and determine its chemistry. It proved to be an expensive and difficult metal to work. Not produced again for a quarter century, 50 tons/year are now produced with 90% used as catalysts.
 Element #43 was named Masurium (Ma) after Walter Noddack´s homeland Masurian marshes in Eastern Prussia that had, like the Rhine, protected Germany from invasion during World War I. But the element was not detected again. Their claim of discovery was ridiculed as a careless mistake. Twelve years later, Italians Carlo Perrier and Emilio Segre isolated trace amounts of a short-lived isotope of element #43 created from Molybdenum irradiated in a Berkeley cyclotron. Perrier and Segre named the element Technetium and were credited with producing the first synthetic element. Tacke's claim was further dishonored.
Element #43 was named Masurium (Ma) after Walter Noddack´s homeland Masurian marshes in Eastern Prussia that had, like the Rhine, protected Germany from invasion during World War I. But the element was not detected again. Their claim of discovery was ridiculed as a careless mistake. Twelve years later, Italians Carlo Perrier and Emilio Segre isolated trace amounts of a short-lived isotope of element #43 created from Molybdenum irradiated in a Berkeley cyclotron. Perrier and Segre named the element Technetium and were credited with producing the first synthetic element. Tacke's claim was further dishonored.
In 1934 Ida Tacke Noddack was the first to suggest that neutron bombardment might cause fission of the uranium nucleus. But her suggestion was ignored. Enrico Fermi´s colleague Emilio Segre, wrote in 1970 The possibility of fission, however, escaped us, although it was called specifically to our attention by Ida Noddack, who sent us an article in which she clearly indicated the possibility of interpreting the results as splitting of the heavy atom into two approximately equal parts. The reason for our blindness is not clear. Fermi said, many years later, that the available data on mass defect at that time were misleading and seemed to preclude the possibility of fission.
Fission was finally discovered by Meitner and Hahn in 1939.
Recently John Armstrong used a sophisticated X-ray database and spectral analysis software to simulate Tacke's team's 1925 data. He found that the spectral lines they attributed to Masurium appear consistent with element #43, supporting their earlier discovery. Additionally, it is now know that an isotope of element #43 occurs naturally in ores containing Uranium. It is likely that this isotope was identified by Tacke's group.
 As early as 1870 Mendeleev had predicted element #87 would be an alkali. But efforts to find it failed because no long-lived isotopes exist. It was discovered in 1939 by Marguerite Perey (1909-1975) at Curie's Institute in Paris. Perey had taken a job in the Institut du Radium when she was 20, becoming Marie Curie's assistant and remained so until Curie died in 1934. In 1939 at the age of 29, while studying isotopes that emit alphas with a range in air greater than 3.5", Perey found that Actinium-227 produces a daughter which decays by beta emission with a half-life of 21 minutes. This new element had the solubility properties of an alkali. The element was first known as Actinium-K but was named Francium (Fr = #87) by Perey in 1946 for her native country. The name and symbol were accepted by the International Union of Chemists (IUC) in 1949. Francium can also be produced by bombarding Thorium with protons. The most stable isotope has a half-life of 22 minutes. In 1949, Perey was appointed to the faculty of the Université of Strasbourg and founded a laboratory for students and colleagues. In 1958 it became the Laboratiore de Chimie Nucléaire of the Centre de Recherches Nucléaires; Perey served as Director.
As early as 1870 Mendeleev had predicted element #87 would be an alkali. But efforts to find it failed because no long-lived isotopes exist. It was discovered in 1939 by Marguerite Perey (1909-1975) at Curie's Institute in Paris. Perey had taken a job in the Institut du Radium when she was 20, becoming Marie Curie's assistant and remained so until Curie died in 1934. In 1939 at the age of 29, while studying isotopes that emit alphas with a range in air greater than 3.5", Perey found that Actinium-227 produces a daughter which decays by beta emission with a half-life of 21 minutes. This new element had the solubility properties of an alkali. The element was first known as Actinium-K but was named Francium (Fr = #87) by Perey in 1946 for her native country. The name and symbol were accepted by the International Union of Chemists (IUC) in 1949. Francium can also be produced by bombarding Thorium with protons. The most stable isotope has a half-life of 22 minutes. In 1949, Perey was appointed to the faculty of the Université of Strasbourg and founded a laboratory for students and colleagues. In 1958 it became the Laboratiore de Chimie Nucléaire of the Centre de Recherches Nucléaires; Perey served as Director.
Starting just before World War II the discovery of elements turned from a search within natural sources to attempts to create new elements that do not presently exist on earth by fusing a small projectile ion onto a large target atom. Three centers were developed for such efforts: University of California-Berkeley in the United States, Dubnia north of Moscow in Russia, and GSI at Darnstad, Germany.
Americium (Am = #95) was isolated in 1945 by Seaborg, Ghiorso, Thompson working in Chicago as part of the Manhattan Project. It was produced under their supervision in a Berkeley cyclotron and in several nuclear reactors about the United States. Elements #93 to #96 did not have the expected properties similar to elements #75 to #78. Seaborg realized that these transuranium elements formed a new series similar to the rare earths. So he named element #95 after the continent of its discovery in the same manner as the element he now considered its homologue: Europium (#63).
Berkelium (Bk = #97) was made in December 1949 at the University of California-Berkeley by Thompson, Ghiorso, and Seaborg by bombarding microgram amounts of Americium with 35MeV Helium ions using the Berkeley 60" cyclotron.
Californium (Cf = #98) was made in January 1950 at the University of California-Berkeley by Thompson, Street, Ghiorso, and Seaborg. Californium was produced by bombarding microgram amounts of Curium with Helium ions.
Dubnium (Db = #105) was made in 1967 both at Berkeley and at the Russian Nuclear Institute at Dubna north of Moscow. Dubium was produced at Dubnia by bombarding Americium with Neon ions and at Berkeley using a Californium target for Nitrogen ions.
Hassium (Hs = #108) was named for the German State of Hesse (Latin for Germany: Hassias) where it was made. Made in January 1984 at Gesellschaft für Schwernonenforschung (GSI) in Darnstad, Germany by P. Armbruster, and G. Münzenerg. Hassium was produced by bombarding Lead with Iron ions.
In June 2001 a team from Lawrence Berkeley Laboratory, the Paul Scherrer Institute in Villigen and the University of Bern, Switzerland, and the Institute of Nuclear Chemistry in Mainz, Germany, reported the results of their experiment where they created Hassium using the GSI UNILAC by
Darmstadtium (Ds = #110): On 9 November 1994 at 4:39 PM the first atom of the heaviest chemical element with atomic number 110 was detected at GSI in Darmstadt, Germany. The element was the subject of a ten year search by many laboratories. International scientists lead by Sigurd Hofmann (on left) and Peter Armbruster (on right) to made and detected the element:  [V.Ninov, F.P.Heβberger, H.Folger, G.Münzenberg, H.J.Schött of GSI; A.G.Popeko, A.V.Yeremin, A.N.Andreyev of Flerov Laboratory, Dubna, Russia; S.Saro, R.Janik of Comenius University, Bratislava, Slovakia, and M.Leino of University of Jyväskylä Jyväskylä, Finland] The element was produced by fusing Nickel and Lead together. This was achieved by accelerating many billion billion Nickel ions to a very specific high velocity in the heavy ion accelerator, UNILAC, at a Lead target.
[V.Ninov, F.P.Heβberger, H.Folger, G.Münzenberg, H.J.Schött of GSI; A.G.Popeko, A.V.Yeremin, A.N.Andreyev of Flerov Laboratory, Dubna, Russia; S.Saro, R.Janik of Comenius University, Bratislava, Slovakia, and M.Leino of University of Jyväskylä Jyväskylä, Finland] The element was produced by fusing Nickel and Lead together. This was achieved by accelerating many billion billion Nickel ions to a very specific high velocity in the heavy ion accelerator, UNILAC, at a Lead target.
The single atom of element #110 was produced and immediately evaporated by the energy of the collision, selected by a velocity filter and then captured in a detector system which measured the decay. The energy of the emitted alpha particle served to identify the atom produced.
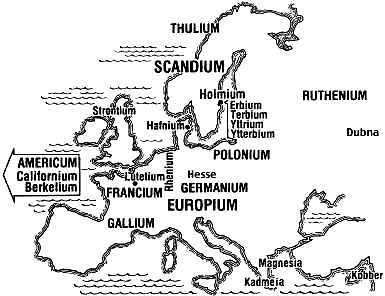
For more elements indicated on the map, see Elements Named for the Locations of Their Minerals and Ores.
![]()
| introduction | alchemy | planets | other celestial objects | color | other properties | myths | people | minerals | ore mines | combination names |
| to site menu | Introduction to Development of Periodic Chart |
18th Century vocabulary, & index of people |
chemistry | physics | ||||||
| created 1 January 2001, amplified 19 Feburary minor revision 16 June 2007 |
by D Trapp | |||||||||