![]()
The ancient Greeks suspected that there must be basic or elementary substances, but they lacked a procedure to determine which substances were elementary. Empedocles selected earth, water, air and fire because they seem to be found in nearly all materials. For example, water is essential for life, and is typically released when materials are heated. Aristotle adopted Empedocles' four earthly elements (and added a fifth, Æther, as the basis of the apparently different heavenly objects). The ancients knew about many other substances, but because they were less common, they were not considered elementary.
 The four earthly elements remained part of accepted theory for over 2000 years. The Greek four elements were incorporated into the arts of alchemy. In their search for formulations for producing desirable substances such as gold, alchemists became convinced that precisely measured proportions are essential.
The four earthly elements remained part of accepted theory for over 2000 years. The Greek four elements were incorporated into the arts of alchemy. In their search for formulations for producing desirable substances such as gold, alchemists became convinced that precisely measured proportions are essential.
Inconsistencies with the expected changes in weight, variations in air produced from diverse source materials, and the realization that vacuums are possible eventually lead to doubts about the four element theory. In his first book Robert Boyle (1627-1691) presented a series of experiments using an air pump (shown behind him at age 37) to create a vacuum. In his second book, Sceptical Chymist (1661) Boyle proposed that an element is certain
primitive and simple, or perfectly unmingled bodies; which not being made of any other body, or of one another, are the ingredients of which all those called perfectly mixt bodies are immediately compounded, and into which they are ultimately resolved.
A century later Antoine-Laurent Lavoisier (1743-1794) explained the advantages of Boyle's proposal considering elements as those substances which are not further separable. The substance's weight would be the key to determine if a change was due to combination or separation. Adding to a substance would increase weight; removal of a component would reduce weight. This new procedure lead Lavoisier to propose a new chemistry
with a revised list of elements. The elements below were all known to ancient cultures, but not thought to be "elementary" prior to Lavoisier.
Lavoisier was raised by a maiden aunt. His father, a wealthy Parisian lawyer provided the best available education at the Collège Mazarin. He learned chemistry from Rouelle who was renown for following Bourdelain's popular chemistry lectures with demonstrations which often did more to show reality varied with theory. In 1766 Lavoisier accompanied a mineral survey of Alsace and Lorraine and won a prize for his essay analyzing methods for lighting a large city. In 1868, at age 25, he was elected to the Académie Royale des Sciences based on his geology work and purchased a partial membership in the Ferme Générale, a firm empowered to collect French taxes. Three years later he married the not quite 14 year-old daughter of another member of the Ferme. (Marie and Antoine shown at right) She became an accomplished linguist helping Lavoisier understand and correspond with English chemists. As his silent partner in science, Marie also drew the sketches for his books and kept his notes.
When in 1775 Lavoisier was appointed régisseur des poudres, they moved into the Arsenal, set up a laboratory, and following their study of the quality of saltpeter, improved French gunpowder from the worst in Europe to the best. Here they met with scientific leaders from Europe and America (Jefferson and Franklin), experimented with and weighed combustion of diamond, sulfur, and phosphorus, calcination of metals and the connection with respiration, and formulated the revolutionary chemistry.
By 1789 French economic instability left Lavoisier little time to continue research. Lavoisier had devoted much of his life to public service. He had reformed the salt tax, instituted uniform moisture to tobacco to make it less brittle, reduced smuggling by having a wall built around Paris, written reports encouraging prison reform and hospital reform, experimented with methods to improve French agriculture and helped found the Society of Agriculture. These and his association with the Académic des Sciences and his work on developing a logical metric system to replace chaotic regional systems or measurement antagonized men like Jean Paul Marat who wrote pamphlets of half truths against learned societies of the aristocracy which had excluded him. Lavoisier was accused and found guilty of ruining air quality with the city wall, of adulterating tobacco with water, and transferring powder from the Arsenal at a time that endangered public safety. Lavoisier was guillotined on May 8, 1794.
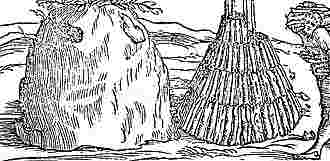 Carbon in the forms of charcoal and soot must have been known to the earliest humans. In Roman times charcoal was made by the same chemistry as it is today, by heating wood in a pyramid covered with clay to exclude air. (The woodcut shows two stages in the manufacture of wood charcoal.) In 1704 Sir Isaac Newton proposed that
diamonds must be combustible. In 1772 Lavoisier demonstrated that charcoal, graphite, and diamond contain the same substance. He demonstrated that a strongly heated diamond sealed from air by clay loses no weight. When heated in a bell jar with air over water or mercury a diamond loses weight, air dimishes 12% by volume, and fixed air (CO2) is produced. Thus the destruction of a diamond, as with other forms of this substance, is
combustion. Lavoisier called the element carbone to distinguish it from charbon (French) for charcoal. Carbon (C = #6): (Latin) = Carbonis (Greek) = charcoal (English). Smithson Tennant confirmed in 1797 that diamonds are solely Carbon by combining a
weighed diamond with saltpeter in a Gold tube.
Carbon in the forms of charcoal and soot must have been known to the earliest humans. In Roman times charcoal was made by the same chemistry as it is today, by heating wood in a pyramid covered with clay to exclude air. (The woodcut shows two stages in the manufacture of wood charcoal.) In 1704 Sir Isaac Newton proposed that
diamonds must be combustible. In 1772 Lavoisier demonstrated that charcoal, graphite, and diamond contain the same substance. He demonstrated that a strongly heated diamond sealed from air by clay loses no weight. When heated in a bell jar with air over water or mercury a diamond loses weight, air dimishes 12% by volume, and fixed air (CO2) is produced. Thus the destruction of a diamond, as with other forms of this substance, is
combustion. Lavoisier called the element carbone to distinguish it from charbon (French) for charcoal. Carbon (C = #6): (Latin) = Carbonis (Greek) = charcoal (English). Smithson Tennant confirmed in 1797 that diamonds are solely Carbon by combining a
weighed diamond with saltpeter in a Gold tube.
Gold nuggets are found naturally in stream-beds because Gold is more dense (19 g/cm3) than most soils. Gold ornaments have been found in prehistoric tombs. The early books of the Bible describe using Gold as a medium of exchange (money). The name Gold (Anglo-Saxon) is related to yellow, which in Anglo-Saxon was called geolo; that derived from jval
(Sanskrit) meaning to shine.
Aurum (Au = #79) came from hari (sanskrit) meaning yellow. Aurora was the goddess of dawn.
Silver rarely occurs uncombined in nature so its discovery and use followed Gold. Silver was rarer and more costly than Gold in Eqypt between the 13th and 15th centuries BC. But by the time the Phoenicians made their first voyage to Spain, they found silver abundant. Silver = Silfr (Norse) and soelfor (Anglo-Saxon) have unknown origins. Argentum (Ag = #47): (Latin) originates from argunas: (Sanskrit) meaning shining.
Copper is found as a native metal in Eqypt and other locations, and can be made from malachite ore by a simple process. Copper (Cu = #29): Kyprion (Greek) = cuprum (Latin) The ore aes cyprium was named after Cypern where malachite was obtained. Cypern bears its name after the cypress tree called Kyparissos.
Iron was probably made by Egyptians and Hittites about 3000 B.C. Furnaces were used to smelt Iron but the ancient processes were kept secret. About 1200 B.C. the Hittite Empire collapsed and iron workers dispersed spreading the technology and starting the Iron Age. The English name Iron = Iren (Anglo-Saxon) is of uncertain origin. Ferrum (Fe = #26) Latin name but may be from earlier Hebrew or Arabic.
Lead ores are widely distributed and easily smelted. The Romans used Lead for water-pipes, writing tablets, coins, and cooking utensils. Lead poisoning was frequent but poorly understood in the Roman civilization. The English name Lead is of unknown origin, but perhaps related to lodd (Norse) and Lot (Germanic). Plumbum (Pb = #82): Lead was called plumbum nigrum (black lead
) by the Romans to distinguish it from plumbum candidum (light lead,
now called Tin). Plumbum (Latin) is possibly related to Molybdos (Greek) also meaning lead. In Scandinavian languages and German, lead is called bly or Blei, words originating from bhlie (Indo-European) meaning shine.
Tin containing bronzes were being made 3000BC, perhaps before the discovery of metallic Tin. Julius Caesar noted production of Tin in the midland regions of Britain. In the first century AD the Romans referred to Tin as plumbum album (white lead
) to distinguish it from Lead which was called plumbum nigrum (black lead
). Pliny wrote that the best (mirrors) known to our forefathers were made at Brundisium from a mixture of Copper and stagnum.
The English name Tin is of unknown origin, perhaps tina (Germanic) for shiny little stick. The Latin name Stannum: (Sn = #50) is connected to stagnum and stag (Indo-European) for dripping because Tin melts easily.
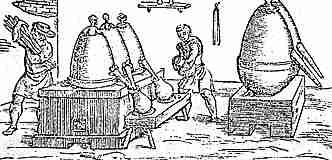
Mercury was known to ancient Chinese, Hindus, and Egyptians. The native ore cinnabar (HgS) was used as a colored pigment, vermilion, and heated or rubbed with vinegar in a brass mortar and pestle to produce the quicksilver. (The woodcut shows Mercury stills, 1540AD.) The name for the liquid metal, Mercury, is from the easily flowing Roman god of messengers and the fast moving planet, both of the same name. Hg (# = 80): Hydrargyrum from hydro-argyros (Greek) for water-silver since mercury is a shiny liquid.

Platinum, like Gold, can be found as grains and nuggets in alluvial sands because it is more dense (21 g/cm3) than most soils. But unlike Gold, Platinum could not be melted by any primitive source of heat. So Platinum had little use until it could be combined and shaped by melting. Platinum (Pt = #78): Plata (Spanish) for silver, -ina is a diminutive suffix. Platinum looks like silver. Platinum was used by South Americans long before Columbus. The use of Platinum was imported to Europe from South America by the Spanish.

Sulfur must have been know to ancient neighbors of natural deposits. Pliny the Elder (Roman) described Italian and Sicilian deposits and medicinal uses, bleaching cloth with Sulfur vapors, and manufacture of Sulfur matches and lamp-wicks. Georgius Agricola (1494-1555 above left) in De Re Metallica described matches ignited by friction on stone and the use of Sulfur in the manufacture of gunpowder. (The woodcut shows distillation of Sulfur, 1557AD.) Early alchemists thought Sulfur was responsible for combustion and therefore must be related to the element fire. The influential alchemist Abu Musa Jabir ibn Hayyan suggested that metals were compounds of Sulfur and Mercury. This made Mercury and Sulfur more important substances to alchemists than other materials. The name Sulfur (S = #16): Schwefel-/svovel/svavl (German & Scandinavian) originated from suelphlos (Indo-European), which is derived from swel meaning to burn slowly.
![]()
| introduction | planets | other celestial objects | color | other properties | myths | people | minerals | ore mines | other places | combination names |
| to site menu | Introduction to Development of Periodic Chart |
18th Century vocabulary, & index of people |
chemistry | physics | ||||||
| created 31 December 2000, amplified 24 Feburary 2001 latest revision 16 June 2007 |
by D Trapp | |||||||||