Contents: #33 As, #41 Cb (Nb), #27 Co, #41 Nb (Cb), #28 Ni, #61 Pm, #73 Ta, #90 Th, #22 Ti, #23 V, #74 W
Contents: #33 As, #41 Cb (Nb), #27 Co, #41 Nb (Cb), #28 Ni, #61 Pm, #73 Ta, #90 Th, #22 Ti, #23 V, #74 W
 Arsenic (As = #33): Arsenikos (Greek) means brave, male. The alchemists related metals to gender. Copper objects were made more masculine (harder and stronger) by adding arsenic as early as 2000 BC. Adsorption of Arsenic caused loss of life to many of the slave miners. But the Greeks and Romans had a different concept of element. Their
Arsenic (As = #33): Arsenikos (Greek) means brave, male. The alchemists related metals to gender. Copper objects were made more masculine (harder and stronger) by adding arsenic as early as 2000 BC. Adsorption of Arsenic caused loss of life to many of the slave miners. But the Greeks and Romans had a different concept of element. Their Arsenic
were sulfide ores, orpiment and sandarac rather than the elemental metal. The first isolation of the metal element is unknown. The German Dominican scholar and alchemist, Albertus Magnus (1193-1280 at right→), in his book De Mineralibus described obtaining the metal by heating orpiment with soap. This and all other metals were considered compounds until Antoine-Laurent Lavoisier (1743-1794) established a new definition for elements.
As there are six kinds of metals, so I have also shown with reliable experiments... that there are also six kinds of half-metals: a new half-metal, namely Cobalt regulusin addition to Mercury, Bismuth, Zinc, and the reguluses of Antimony and Arsenic. He gave six ways to distinguish Bismuth and Cobalt which were typically found in the same ores:
German miners found a dense, reddish brown ore frequently covered with green spots. Much like Kobalt, this ore was found useful for coloring glass green. The ore looked like Copper ore, but contained no Copper. Miners called it Kupfer-nickel meaning devil copper. Nickel (German) means devil or deceptive little spirits. Nickel (Ni = #28): In 1751 Axel Fredrik Cronstedt, (1722-1765) a Swedish metallurgist in the Bureau of Mines, investigated a new mineral sample. He placed some Iron in the acid solution of the ore hoping to see Copper deposit. When no deposit formed he then reduced the green crystals with charcoal obtaining a while metal, slightly magnetic, and different from others. He described it as a half-metal and chose to retain the name Nickel. Like all metals, Nickel was not considered an element until Lavoisier (1743-1794) proposed his new chemistry. Cronstedt also developed an excellent classification of minerals that was translated to several languages. In 1756 he discovered a zeolite now widely used for water softening.

 A white mineral often accompanied tin ore making it difficult to smelt tin without producing a dirt-like impurity. It was named wolframite because it possessed the story-book evil of the wolf (German) and produced the dirt-like tin impurity, rahm (German for dirt). In 1761 J. G. Lehmann fused it with sodium nitrate and found the melt to dissolve in water forming a green solution which turned red (due to manganate and permanganate). Adding mineral acid (H2SO4) precipitated a white spongy earth which turned yellow after long standing. In 1779 Peter Woulfe cooked wolframite in the acid of salt (HCl) and upon finding a rich yellow color suggested it might contained something new. Meanwhile, a white mineral which had been called tungsten [tung (Swedish) for heavy and sten (Swedish) for stone] was found by Carl Scheele (←at left) in 1781 to contain lime (CaO) and an acidic white powder after decomposition with aqua fortis (HNO3). Tungsten [CaWO4] subsequently became known as scheelite. Scheele proposed that some of its constituents probably remained unknown. Torbern Bergman, believing scheelite's high density suggested it contained the heavy earth baryta, was frustrated when he too found it contained the acidic material rather than the alkaline expected for baryta. He proposed that it might be possible to prepare a metal from the acid. Meanwhile two Spanish chemists, Don Juan José d'Elhuyar y de Zubice and his younger brother Don Fausto d'Elhuyar (1755-1833 at right→) studied respectively metallurgy and mineralogy about Europe; Don Juan José studied a half year with Bergman in Upsala and visited Scheele. After returning to Vergara, the brothers in 1783 analyzed wolframite [(Fe,Mn)WO4] and found it contained the same acid as tungsten. They then produced the new metal by reducing the acid by strongly heating with powdered charcoal. Much of the world calls the metal as they did, Wolfram (W = #74) after the German origin. Others call the metal Tungsten after the Swedish origin. The Spanish discoverers noted that there was no Spanish name! In order to establish mining in the New World, Don Juan José was sent to New Granada (Colombia) where he worked until his death in 1804 in the Santa Ana mine at Bogotá. Don Fausto was sent to Mexico City in 1788 and spent 33 years as Director General of Mines until the Mexican war of independence; he established the school of mines and investigated cold amalgamation for obtaining Silver from ores. He was famous in Spain and continued to be honored by celebrations 100 years after his death.
A white mineral often accompanied tin ore making it difficult to smelt tin without producing a dirt-like impurity. It was named wolframite because it possessed the story-book evil of the wolf (German) and produced the dirt-like tin impurity, rahm (German for dirt). In 1761 J. G. Lehmann fused it with sodium nitrate and found the melt to dissolve in water forming a green solution which turned red (due to manganate and permanganate). Adding mineral acid (H2SO4) precipitated a white spongy earth which turned yellow after long standing. In 1779 Peter Woulfe cooked wolframite in the acid of salt (HCl) and upon finding a rich yellow color suggested it might contained something new. Meanwhile, a white mineral which had been called tungsten [tung (Swedish) for heavy and sten (Swedish) for stone] was found by Carl Scheele (←at left) in 1781 to contain lime (CaO) and an acidic white powder after decomposition with aqua fortis (HNO3). Tungsten [CaWO4] subsequently became known as scheelite. Scheele proposed that some of its constituents probably remained unknown. Torbern Bergman, believing scheelite's high density suggested it contained the heavy earth baryta, was frustrated when he too found it contained the acidic material rather than the alkaline expected for baryta. He proposed that it might be possible to prepare a metal from the acid. Meanwhile two Spanish chemists, Don Juan José d'Elhuyar y de Zubice and his younger brother Don Fausto d'Elhuyar (1755-1833 at right→) studied respectively metallurgy and mineralogy about Europe; Don Juan José studied a half year with Bergman in Upsala and visited Scheele. After returning to Vergara, the brothers in 1783 analyzed wolframite [(Fe,Mn)WO4] and found it contained the same acid as tungsten. They then produced the new metal by reducing the acid by strongly heating with powdered charcoal. Much of the world calls the metal as they did, Wolfram (W = #74) after the German origin. Others call the metal Tungsten after the Swedish origin. The Spanish discoverers noted that there was no Spanish name! In order to establish mining in the New World, Don Juan José was sent to New Granada (Colombia) where he worked until his death in 1804 in the Santa Ana mine at Bogotá. Don Fausto was sent to Mexico City in 1788 and spent 33 years as Director General of Mines until the Mexican war of independence; he established the school of mines and investigated cold amalgamation for obtaining Silver from ores. He was famous in Spain and continued to be honored by celebrations 100 years after his death.
The Reverend William Gregor was born in Cornwall, England, in 1761 and educated for the ministry at Bristol and Cambridge. He became interested in minerals and was acknowledged as greatly skilled by Berzelius. He analyzed a number of substances such as topaz. One was a black magnetic sand from the Menachan valley in his own parish. His analysis was published in Crell's Annalen in 1791: The sand is black, and in external appearance resembles gunpowder. Its grains are of various sizes, but have no definite shape.
It had the composition:
that menachanite has for its constituent parts Iron, and a peculiar metallic oxyd of an unknown nature. By the following examination it will appear that this substance, which besides iron, forms the second chief component principle of menachanite, is precisely the very same which constitutes the Hungarian red schörl.Klaproth gave the following reason for his name of the new element within the oxide:
Whenever no name can be found for a new fossil which indicates its peculiar and characteristic properties (in which situation I find myself at present) I think it best to choose such a denomination as means nothing of itself, and thus can give no rise to any erroneous ideas. (Lavoisier had suggested similar precautions for naming new elements.) In consequence of this, as I did in the case of Uranium, I shall borrow the name for this metallic substance from mythology, and in particular from the Titans, the first sons of the earth. I therefore call this new metallic genus Titanium.(Ti = #22): The Titans, the giants, were the first gods in Greek mythology. The first Titan, Gaea (the Earth) gave birth to Uranos (the sky). But the actual isolation of the pure element proved elusive. Klaproth, Vauquelin, and Heinrich Rose tried unsuccessfully. Berzelius prepared some very impure amorphous Titanium in 1825. Wöhler tried to exclude air in 1849 but probably produced the nitride. Lars Fredrik Nilson and Otto Pettersson prepared 95% pure Titanium in 1887 reducing the tetra chloride with Sodium in an air tight steel cylinder. Moissan used his electric furnace to produce 98% purity. M.A.Hunter produced 99.9% pure Titanium in 1910. Today Titanium is used in steel to prevent bubbles which would weaken castings. Rutile, TiO2, is widely used as a high grade white pigment. Because of its high index of refraction of light, it is very white and very opaque.

 The first governor of Connecticut, John Winthrop (the younger) (1606-1676 at right→), was an alchemist, manufacturing chemist, physician, and rock collector. He picked up a rock called columbite near New London, Connecticut, which his grandson sent to the British Museum in London (perhaps on the governor's death). Decades later Charles Hatchett (1765-1847 ←at left) noted the columbite in the Museum's collection and analyzed it. While the columbite is a very complex mineral, Hachett noted in 1801 that it contained a new earth (which in turn implied that it contained a new element, called Columbium (Cb = #41): Columbia derives from Christopher Columbus but may have been intended to mean
The first governor of Connecticut, John Winthrop (the younger) (1606-1676 at right→), was an alchemist, manufacturing chemist, physician, and rock collector. He picked up a rock called columbite near New London, Connecticut, which his grandson sent to the British Museum in London (perhaps on the governor's death). Decades later Charles Hatchett (1765-1847 ←at left) noted the columbite in the Museum's collection and analyzed it. While the columbite is a very complex mineral, Hachett noted in 1801 that it contained a new earth (which in turn implied that it contained a new element, called Columbium (Cb = #41): Columbia derives from Christopher Columbus but may have been intended to mean from America.
Later Hatchett gave up chemical analysis to devote full time to making money at the family coach fabrication business.
 Anders Gustaf Ekeberg (1767-1813 ←at left) was born in Stockholm to a ship-builder, sent to boarding schools at age 10, found pleasure in Greek literature, and graduated from the University of Upsala in 1788 with a thesis on
Anders Gustaf Ekeberg (1767-1813 ←at left) was born in Stockholm to a ship-builder, sent to boarding schools at age 10, found pleasure in Greek literature, and graduated from the University of Upsala in 1788 with a thesis on Oils Extracted from Seeds.
After travelling through Germany he returned to a teaching career at Upsala where he taught, wrote poetry, presented chemical expositions, and analyzed minerals. Ekeberg was handicapped by partial deafness from a childhood illness and blinded in one eye when a flask he was holding exploded. He was fascinated by the minerals of Ytterby and Fahlun. In 1802 he analyzed specimens from Kimito, Finland, and yttrotantalite from Ytterby, finding both contained the same new metal. Ekeberg called it Tantalum (Ta = #73). Tantalus, son of Jupiter by Greek myth, was condemned to hell, standing to his neck in water. But the water sank when he stooped to drink. Ta2O5 does not take in water,
nor dissolve in acids. Furthermore, Ekeberg had found the task of finding the element tantalizing.
 In 1809 Wollaston analyzed columbite and tantalite and concluded their metals were identical. This was accepted until 1846 when Heinrich Rose (←at left, son of a student of Klaproth) distinguished the two metals. Columbium forms valences of 3 and 5, whereas Tantalum always has a valence of 5. While it continued to be known as Columbium in the United States for some time, in Europe the metal was called Niobium (Nb = #41) as named by Heinrich Rose. Niobe was the daughter of Tantalus in Greek mythology. Minerals that contain Tantalum invariably also contain Niobium since they have very similar chemical properties.
In 1809 Wollaston analyzed columbite and tantalite and concluded their metals were identical. This was accepted until 1846 when Heinrich Rose (←at left, son of a student of Klaproth) distinguished the two metals. Columbium forms valences of 3 and 5, whereas Tantalum always has a valence of 5. While it continued to be known as Columbium in the United States for some time, in Europe the metal was called Niobium (Nb = #41) as named by Heinrich Rose. Niobe was the daughter of Tantalus in Greek mythology. Minerals that contain Tantalum invariably also contain Niobium since they have very similar chemical properties.
 Ekeberg's later years were made difficult by continuing illness. His analysis of the mineral water of Medevi was assisted by a student who was to become a far more influential discovery that the element Tantalus. Jöns Jakob Berzelius (1779-1849 at right 1823→) caused his teachers concern when his interest in science failed to extend to other required university courses. But he graduated from Upsula, took a hospital job, and in time became a professor. He devoted spare time to chemistry and in 1808 published a textbook which went through five editions and was translated to German and French. By 1810 he began publishing studies on combining proportions of Lavoisier's new elements. He understood the value of the atoms proposed by Dalton and realized the significance of accurate atomic weights. He often repeated analysis many times before he was satisfied by consistent results. In 1813 he proposed chemical symbols using the initials of their Latin names which was slowly adopted and continues in use today. In 1822 he began publishing Jahres-Bericht, an annual review which reported his researches and those of others he considered most significant. His manual on blowpipe analysis was the standard for years. Berzelius made several long trips about Europe, becoming acquainted with the important scientists. His wide correspondence kept him in touch with scientific progress everywhere. His private laboratory was occasionally opened to students and colleagues who sought his expertise. Many of their accomplishments are described elsewhere in this history.
Ekeberg's later years were made difficult by continuing illness. His analysis of the mineral water of Medevi was assisted by a student who was to become a far more influential discovery that the element Tantalus. Jöns Jakob Berzelius (1779-1849 at right 1823→) caused his teachers concern when his interest in science failed to extend to other required university courses. But he graduated from Upsula, took a hospital job, and in time became a professor. He devoted spare time to chemistry and in 1808 published a textbook which went through five editions and was translated to German and French. By 1810 he began publishing studies on combining proportions of Lavoisier's new elements. He understood the value of the atoms proposed by Dalton and realized the significance of accurate atomic weights. He often repeated analysis many times before he was satisfied by consistent results. In 1813 he proposed chemical symbols using the initials of their Latin names which was slowly adopted and continues in use today. In 1822 he began publishing Jahres-Bericht, an annual review which reported his researches and those of others he considered most significant. His manual on blowpipe analysis was the standard for years. Berzelius made several long trips about Europe, becoming acquainted with the important scientists. His wide correspondence kept him in touch with scientific progress everywhere. His private laboratory was occasionally opened to students and colleagues who sought his expertise. Many of their accomplishments are described elsewhere in this history.
In 1815 Berzelius found in a rare mineral from the Fahlun district what was apparently an oxide of a new metal. He named the metal Thorium after Thor, the Norse god of war. But ten years later Berzelius himself determined the mineral matched the properties of yttrium phosphate so did not contain any new element. In 1829 Berzelius analyzed a rock sent him by pastor Esmarck from the Norwegian island of Lövö near Brevig. The black rock with no texture or crystalline form looked like gadolinite from Ytterby. Its high density led Esmarck to suspect it might contain Tantalum. Berzelius heated a mixture of the mineral and potassium (a procedure previously used to discover three elements: Titanium, Cerium, Zirconium) finding another new metal. Berzelius reused the name Thorium (Th = #90). The mineral later was called thorite. In 1898 Marie Curie in Paris and G.C. Schmidt of the University of Münster independently found that Thorium is radioactive. It is the parent of a long series of elements produced by radioactive decay.
 In 1801 Andrés Manuel del Río, (1764-1849) a professor of mineralogy in Mexico (presumably under Don Fausto d'Elhuyar) examined brown lead from Zimapán and conclude it contained a new metal similar to Chromium and Uranium. Because of the red color that its salts acquire on heating, he called it Erythronium. Before publishing, he decided he was mistaken, and it was a lead chromate. In 1805 Collet-Descotils confirmed del Río's analysis as lead chromate. del Río who was born in Madrid and studied at Freiberg, continued teaching for about 50 years at the Mexican School of Mines, and during the Mexican war of independence plead for Mexico in the Spanish court. But in 1831 Nils Gabriel Sefström (1787-1845 ←at left), physician and chemistry professor at the Fahlun School of Mines (100 miles northwest of Stockholm) found a remarkably soft iron from the Taberg mine that when tested with muriatic acid (HCl) gave a black powder which usually indicated that the iron would be brittle. Bringing a large sample of the black powder to Stockholm, Sefström and Berzelius investigated for three weeks finding many common elements including a new substance. After Sefström's departure, Berzelius continued to determined many additional properties. Sefström and Berzelius named the element Vanadium (V = #23) because of the multicolored compounds. Vanadis, is a nickname for Freya, the Norse goddess of beauty. Wöhler, chided by Berzelius for investigating del Río's ore but missing the discovery, confirmed that a Vanadium sample Berzelius forwarded was identical to Erythronium. Sir Henry Enfield Roscoe (1833-1915), who passed his doctor's examination summa cum laude while assisted Robert Bunsen in Heibelberg in the famous researches on spectra of elements, later isolated and identified many oxidation states of Vanadium and in 1869 finally isolated metallic Vanadium.
In 1801 Andrés Manuel del Río, (1764-1849) a professor of mineralogy in Mexico (presumably under Don Fausto d'Elhuyar) examined brown lead from Zimapán and conclude it contained a new metal similar to Chromium and Uranium. Because of the red color that its salts acquire on heating, he called it Erythronium. Before publishing, he decided he was mistaken, and it was a lead chromate. In 1805 Collet-Descotils confirmed del Río's analysis as lead chromate. del Río who was born in Madrid and studied at Freiberg, continued teaching for about 50 years at the Mexican School of Mines, and during the Mexican war of independence plead for Mexico in the Spanish court. But in 1831 Nils Gabriel Sefström (1787-1845 ←at left), physician and chemistry professor at the Fahlun School of Mines (100 miles northwest of Stockholm) found a remarkably soft iron from the Taberg mine that when tested with muriatic acid (HCl) gave a black powder which usually indicated that the iron would be brittle. Bringing a large sample of the black powder to Stockholm, Sefström and Berzelius investigated for three weeks finding many common elements including a new substance. After Sefström's departure, Berzelius continued to determined many additional properties. Sefström and Berzelius named the element Vanadium (V = #23) because of the multicolored compounds. Vanadis, is a nickname for Freya, the Norse goddess of beauty. Wöhler, chided by Berzelius for investigating del Río's ore but missing the discovery, confirmed that a Vanadium sample Berzelius forwarded was identical to Erythronium. Sir Henry Enfield Roscoe (1833-1915), who passed his doctor's examination summa cum laude while assisted Robert Bunsen in Heibelberg in the famous researches on spectra of elements, later isolated and identified many oxidation states of Vanadium and in 1869 finally isolated metallic Vanadium.
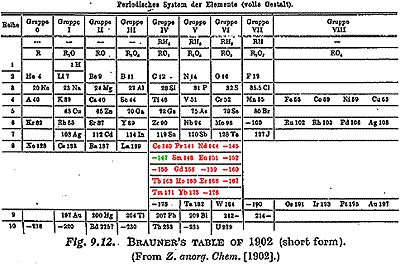 In 1902 the Czech chemist Bohuslav Brauner addressed the lack of positions for rare earths on the period chart of Mendeleev by extending the chart downward after Lanthanum. Brauner arguing that several discontinuities of properties are apparent on his new periodic chart (colors added), and predicted the existence of an element in between Neodynium and Samarium. Henry Moseley's study of X-ray spectra confirmed an element was missing at atomic #61. A search of minerals containing adjoining elements in the 1920s brought claims of discovery from Florence and New Hampshire, and Illinois with names of Florentium and Illinium. All had support of competent X-ray spectroscopists. But W. Prandtl and the Noddacks were unable to confirm the new element. In 1938 L.L. Quill and colleagues used the new Ohio State cyclotron to bombard Neodymium and Sumarium with various projectiles. A number of radioactive isotopes were produced, presumably including one due to element #61 for which they proposed the name Cyclonium.
In 1902 the Czech chemist Bohuslav Brauner addressed the lack of positions for rare earths on the period chart of Mendeleev by extending the chart downward after Lanthanum. Brauner arguing that several discontinuities of properties are apparent on his new periodic chart (colors added), and predicted the existence of an element in between Neodynium and Samarium. Henry Moseley's study of X-ray spectra confirmed an element was missing at atomic #61. A search of minerals containing adjoining elements in the 1920s brought claims of discovery from Florence and New Hampshire, and Illinois with names of Florentium and Illinium. All had support of competent X-ray spectroscopists. But W. Prandtl and the Noddacks were unable to confirm the new element. In 1938 L.L. Quill and colleagues used the new Ohio State cyclotron to bombard Neodymium and Sumarium with various projectiles. A number of radioactive isotopes were produced, presumably including one due to element #61 for which they proposed the name Cyclonium.
 Isolation and identification of element #61 was finally made by (shown from left to right) Jack A. Marinsky, Lawrence E. Glendenin and Harold G. Richter working with Charles D. Coryell separating fission fragments during World War-II. They precipitated Cerium from the rare earth fraction as an iodate, Yttrium, Samarium, and Europium by digestion with carbonate, then separated the rest using an Amberlite ion exchange column. Praseodymium, Neodymium, element #61, and Yttrium were adsorbed at different levels and eluted with ammonium citrate at various pHs. Element #61 emitted beta rays with a 3.7-year half life. 14761 was confirmed by mass spectrograph. The most stable isotope currently known has a half-life of 25 years, too short to be in any of the minerals investigated in the 1920s. Grace Mary Coryell suggested the name Prometheum (Pm = #61): Prometheus was the god who stole fire from heaven. He gave it to humans and was daily punished by Zeus. Since Promethium does not exist except in fission products, it was named for the courage and pain needed to synthesize new elements. In 1949 the International Union of Chemistry adopted the spelling Promethium.
Isolation and identification of element #61 was finally made by (shown from left to right) Jack A. Marinsky, Lawrence E. Glendenin and Harold G. Richter working with Charles D. Coryell separating fission fragments during World War-II. They precipitated Cerium from the rare earth fraction as an iodate, Yttrium, Samarium, and Europium by digestion with carbonate, then separated the rest using an Amberlite ion exchange column. Praseodymium, Neodymium, element #61, and Yttrium were adsorbed at different levels and eluted with ammonium citrate at various pHs. Element #61 emitted beta rays with a 3.7-year half life. 14761 was confirmed by mass spectrograph. The most stable isotope currently known has a half-life of 25 years, too short to be in any of the minerals investigated in the 1920s. Grace Mary Coryell suggested the name Prometheum (Pm = #61): Prometheus was the god who stole fire from heaven. He gave it to humans and was daily punished by Zeus. Since Promethium does not exist except in fission products, it was named for the courage and pain needed to synthesize new elements. In 1949 the International Union of Chemistry adopted the spelling Promethium.
![]()
| introduction | alchemy | planets | other celestial objects | color | other properties | people | minerals | ore mines | other places | combination names |
| to site menu | Introduction to Development of Periodic Chart |
18th Century vocabulary, & index of people |
chemistry | physics | ||||||
| created 31 December 2000 amplified 19 May 2001 latest revision 16 June 2007 |
by D Trapp | |||||||||