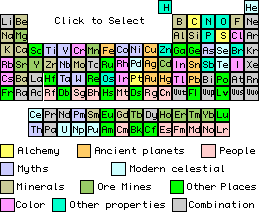
Origins of the Element Names
Names Constructed from other Words

Page 1 Contents: #3 Li, #10 Ne, #18 Ar, #36 Kr, #54 Xe, #57 La, #59 Pr, #60 Nd, #66 Dy, #83 Bi, #86 Rn, #88 Ra, #89 Ac
Page 2 Contents: #43 Tc, #85 At, #91 Pa, #104 Unq=Rf, #105 Unp=Db, #106 Unh=Sg, #107 Uns=Bh, #108 Uno=Hs, #109 Une=Mt, #110 Uun=Ds, #111 Uuu=Rg, #112 Uub, #113 Uut, #114 Uuq, #115 Uup, #116 Uuh, #117 Uus, #118 Uuo
Bi (#83) = Bismuth: Wiese (German) for field
and Muten (German) means to apply for
mineral rights or perhaps Weisse Masse (German) means white mass.
In either case, Bismuth is a Latinized form of the German words. Bismuth was used as early as 1400 as a base metal over layed with gold or amber lacquer. While Gutenberg first printed used brass type, by 1450 type cast from a Bismuth alloy was introduced. Alchemy held that one metal could be transformed to another. Miners believed there were three types of Lead: Ordinary, Tin, and Bismuth. Silver was often found in ore below Bismuth. So they believed that Bismuth had progressed farthest but not completed its transmutation to Silver. Striking a vein of Bismuth, miners would say sadly, Alas, we have come too soon.

 The Greeks had proposed that the
The Greeks had proposed that the earths
were common, fundamental, elementary substances. That view continued for two millennia. Robert Boyle, (←at left) challenging in his Skeptical Chemist the usefulness of the Four Element Theory (Earth, Water, Air and Fire), speculating that a material not separable is more elementary. A century later Antoine Lavoisier (shown with Marie, wife and assistant→) in Paris detailed how Boyle's new definition of element provided a clearer view of nature. But in the laboratory, earths continued to be treated as characteristic of the new elements within. A discovery of a new earth (each earth given a name ending in a
) was regarded as equivalent to discovering the element within.
 The great Swedish chemist Jön Berzelius (←at left) became an ardent supporter of the new chemistry of Lavoisier and the subsequent atomic theory of John Dalton. Johan August Arfwedson (b1792, d1841) was a skillful young chemist studying chemistry under Berzelius. In 1817 he analyzed a rock previously discovered by a Brazilian, José Bonifácio de Andrada e Silva, while touring the Scandinavian island of Utö which is two miles into the Baltic. Several chemists had previously failed to investigate the red color this stone imparted to flames, or the puzzling losses during its analysis. Arfwedson found the rock was roughly 80% silica, 17% alumina, and 3% alkali. He found the alkali did not precipitate in tartaric acid like potassium and was not magnesium. Calculating its composition by presuming it to be soda resulted in a 5% excess. Two careful repetitions convinced Arfwedson that it was a new element which has a greater capacity to react than the other alkalies. Berzelius wrote to Berthollet:
The great Swedish chemist Jön Berzelius (←at left) became an ardent supporter of the new chemistry of Lavoisier and the subsequent atomic theory of John Dalton. Johan August Arfwedson (b1792, d1841) was a skillful young chemist studying chemistry under Berzelius. In 1817 he analyzed a rock previously discovered by a Brazilian, José Bonifácio de Andrada e Silva, while touring the Scandinavian island of Utö which is two miles into the Baltic. Several chemists had previously failed to investigate the red color this stone imparted to flames, or the puzzling losses during its analysis. Arfwedson found the rock was roughly 80% silica, 17% alumina, and 3% alkali. He found the alkali did not precipitate in tartaric acid like potassium and was not magnesium. Calculating its composition by presuming it to be soda resulted in a 5% excess. Two careful repetitions convinced Arfwedson that it was a new element which has a greater capacity to react than the other alkalies. Berzelius wrote to Berthollet: We have given this alkali the name of Lithion to recall that it was discovered in the mineral kingdom, whereas the two others were [discovered] in the vegetable kingdom.
Li = Lithium (#3): Lithos (Greek) means stone. Their attempts to isolate elemental Lithium failed. Humphrey Davy and William Thomas Brande (b1788, d1866, who succeeded Davy at the Royal Institution) obtained small amounts using very powerful batteries in 1818. Bunsen and Matthiessen finally succeeded to prepare enough of the element in 1855 for a thorough study of its properties.
 Carl Gustav Mosander (b1797,d1858, at right→), an assistant of Berzelius, lived the house with Berzelius and his wife for years. Later he had an apartment adjoining the Stockholm mineral collections he tended. He also was professor and had charge of the chemical laboratory for medical students. Father Moses, as Berzelius and their German friend Wöhler called him, partially separated cerite with hot dilute nitric acid. He retained the old name ceria for the insoluble oxide. Berzelius wrote Wöhler on February 12 1839:
Carl Gustav Mosander (b1797,d1858, at right→), an assistant of Berzelius, lived the house with Berzelius and his wife for years. Later he had an apartment adjoining the Stockholm mineral collections he tended. He also was professor and had charge of the chemical laboratory for medical students. Father Moses, as Berzelius and their German friend Wöhler called him, partially separated cerite with hot dilute nitric acid. He retained the old name ceria for the insoluble oxide. Berzelius wrote Wöhler on February 12 1839: Mosander seems willing to take my suggestion to name it (the new element) Lanthanum (La = #57) and the oxide (the new soluble salt) lanthanum oxide or lanthana.
Lanthano (Greek) means to hide or to escape notice.
Lanthana lay hidden in the mineral cerite for 36 years after ceria (containing element Cerium) was discovered in the mineral cerite in 1803. Mosander withheld publication frustrating Berzelius and Wöhler, while continuing his efforts at purification. In 1841 Mosander separated a new rose-colored oxide from lanthana. He called the element within the oxide Didymium. Didymos (Greek) means twin and this oxide has properties similar to its twin
Lanthanum. In 1843 he further showed that yttria from which all the ceria, lanthana, and didymia had been removed, still contained at least three other earths, a colorless oxide for which he kept the name yttria, a yellow earth erbia, and a rose-colored terbia.
 Carl Auer was a quiet, industrious, unsociable boy from Austria who became a favorite student of Bunsen in Heidelberg. When he showed Bunsen a tiny handful of rare earth minerals he had collected from the north, Bunsen laughingly encouraged him to begin a study of them. Auer did so for the rest of his life. A number of chemists believed that Didymium was a mixture of elements but none had made a successful separation. In 1885 at age 26 Auer announced to the Vienna Academy of Sciences that by repeated fractionation of ammonium didymium nitrate, he had succeeded in splinting didymia into two earths. He proposed the names Praseodymium = Pr (#59): Praseios, (Greek) for leek-green since it has greenish salts, and didymos meaning twin and Neodymium = Nd (#60) Neos (Greek) means new.
Carl Auer was a quiet, industrious, unsociable boy from Austria who became a favorite student of Bunsen in Heidelberg. When he showed Bunsen a tiny handful of rare earth minerals he had collected from the north, Bunsen laughingly encouraged him to begin a study of them. Auer did so for the rest of his life. A number of chemists believed that Didymium was a mixture of elements but none had made a successful separation. In 1885 at age 26 Auer announced to the Vienna Academy of Sciences that by repeated fractionation of ammonium didymium nitrate, he had succeeded in splinting didymia into two earths. He proposed the names Praseodymium = Pr (#59): Praseios, (Greek) for leek-green since it has greenish salts, and didymos meaning twin and Neodymium = Nd (#60) Neos (Greek) means new.
Auer also invented the incandescent gas mantle (commonly used in Coleman gas lanterns). Bunsen had found that a poorly burning fuel is responsible for luminous flames. Bunsen designed a burner that completely burns fuel resulting in a nearly colorless flame suitable for spectral analysis. Auer decided that instead of producing light with a poorly burning luminous flame, it would be better to use a completely burning fuel to heat a refractory mantle to glowing. After many attempts, Auer impregnated fabric with a mixture of thorium nitrate, cerium nitrate, beryllium nitrate, magnesium nitrate and water. Kaiser Franz Josef elevated Auer to hereditary nobility with the title of Freiherr von Welsbach. The incandescent lamp is known in Germany as the Auerlicht and in American as the Welsbach mantle.
 In general the lanthanide series was difficult to isolate because they are chemically nearly identical. They occur closely associated in very complex minerals. They have all been obtained by elaborate and laborious fractionations. As a result they are often called rare earths. While some are moderately abundant on earth, their pure elements are rare and costly even in the form of their earths (compounds). In 1886 Lecoq de Boisbaudran started with what was considered
In general the lanthanide series was difficult to isolate because they are chemically nearly identical. They occur closely associated in very complex minerals. They have all been obtained by elaborate and laborious fractionations. As a result they are often called rare earths. While some are moderately abundant on earth, their pure elements are rare and costly even in the form of their earths (compounds). In 1886 Lecoq de Boisbaudran started with what was considered pure
holmia. He fractionally precipitated small amounts of rare earth elements on a marble slab in his fireplace including a new rare earth which he called dysprosia. Using ammonium hydroxide and then saturated potassium sulfate, he successively precipitated compounds of Terbium, Dysprosium, Holmium, and Erbium. He chose the name Dysprosium (Dy = #66) because Dysprositos (Greek) means difficult to attain.
 In 1785 Henry Cavendish thought there might be an unknown gas in the atmosphere when, after passing electric sparks through air, he found a small part of the phlogistonated air (later called Nitrogen) failed to oxidize. The residue was small, not more than 1/120 of the phlogistinated air, and was forgotten as trivial. A century later Robert John Strutt, third Lord Rayleigh, (b1842, d1919, at right→) began an investigation of gas densities. Known for his clear thinking, Lord Rayleigh succeeded upon Clerk Maxwell´s death to Professor at Cavendish Laboratory. He attracted increased enrollment and admitted women for the first time. Rayleigh found that Oxygen was NOT exactly 16 times heavier than Hydrogen, but 15.882. He was puzzled when Nitrogen from ammonia was slightly lighter than that from air. He thought of four possible explanations and was working to eliminate each.
In 1785 Henry Cavendish thought there might be an unknown gas in the atmosphere when, after passing electric sparks through air, he found a small part of the phlogistonated air (later called Nitrogen) failed to oxidize. The residue was small, not more than 1/120 of the phlogistinated air, and was forgotten as trivial. A century later Robert John Strutt, third Lord Rayleigh, (b1842, d1919, at right→) began an investigation of gas densities. Known for his clear thinking, Lord Rayleigh succeeded upon Clerk Maxwell´s death to Professor at Cavendish Laboratory. He attracted increased enrollment and admitted women for the first time. Rayleigh found that Oxygen was NOT exactly 16 times heavier than Hydrogen, but 15.882. He was puzzled when Nitrogen from ammonia was slightly lighter than that from air. He thought of four possible explanations and was working to eliminate each.
 William Ramsay (b1852, d1916, ←at left) was known to sit quietly as a child through long boring sermons; he spent that time learning to read French and German Bibles by comparing their text to the English he had memorized. Ramsay also worked out geometry propositions studying church window designs. He did advanced chemistry and glass blowing in his bedroom. He excelled in individual sports and drew crowds when diving. He gained permission from Lord Rayleigh to investigate atmospheric Nitrogen. He found after removing Nitrogen by hot Magnesium, 1/80 of the volume remained with a density of 19.086 g/cm3. Examining its spectrum, Ramsey noted groups of red and green lines not due to other gases. Exchanging daily letters with Rayleigh, Ramsey wrote on May 24, 1894:
William Ramsay (b1852, d1916, ←at left) was known to sit quietly as a child through long boring sermons; he spent that time learning to read French and German Bibles by comparing their text to the English he had memorized. Ramsay also worked out geometry propositions studying church window designs. He did advanced chemistry and glass blowing in his bedroom. He excelled in individual sports and drew crowds when diving. He gained permission from Lord Rayleigh to investigate atmospheric Nitrogen. He found after removing Nitrogen by hot Magnesium, 1/80 of the volume remained with a density of 19.086 g/cm3. Examining its spectrum, Ramsey noted groups of red and green lines not due to other gases. Exchanging daily letters with Rayleigh, Ramsey wrote on May 24, 1894: Has it occurred to you that there is room for gaseous elements at the end of the first column of the period table?
They jointly presented the discovery of the first inert gas to the British Association at Oxford the same month. The chairman proposed they call it Argon, (Ar: #18) the lazy one,
because Argon is chemically inert. A-ergon (Greek) means no work or no action.
 Joined by an assistant, Morris William Travers (at right→), Ramsay continued to search for another member of the expected inert gas family lighter than Argon. They failed to find such a gas in rare minerals. Given a liter of liquid air, they used it to develop their skills manipulating gases. After most of the N2, O2, and Ar had distilled from liquefied air, they found the remains still contained oxygen and nitrogen which they removed with red-hot copper and magnesium. After lunch May 30, 1898, they observed the spectra of the inert remaining 25 cm3 of the original liter. Unique bright yellow and brilliant green lines suggested a new element which, because it remained hidden in the liquid, they named Krypton (Kr: #36). Kryptos (Greek) means hidden. Working until 11 PM Ramsay and Travers measured the density that placed it between Bromine and Rubidium on the periodic table. Since this was NOT the expected gas lighter than Argon, they continued their search.
Joined by an assistant, Morris William Travers (at right→), Ramsay continued to search for another member of the expected inert gas family lighter than Argon. They failed to find such a gas in rare minerals. Given a liter of liquid air, they used it to develop their skills manipulating gases. After most of the N2, O2, and Ar had distilled from liquefied air, they found the remains still contained oxygen and nitrogen which they removed with red-hot copper and magnesium. After lunch May 30, 1898, they observed the spectra of the inert remaining 25 cm3 of the original liter. Unique bright yellow and brilliant green lines suggested a new element which, because it remained hidden in the liquid, they named Krypton (Kr: #36). Kryptos (Greek) means hidden. Working until 11 PM Ramsay and Travers measured the density that placed it between Bromine and Rubidium on the periodic table. Since this was NOT the expected gas lighter than Argon, they continued their search.
In June, Ramsay and Travers next solidified some of their precious fifteen liters of Argon by surrounding it with liquid air boiling under reduced pressure. They then collected the first of the Argon to vaporize. This had a complex spectra with many lines in red, a number of faint green, and some in violet. The yellow line is fairly bright, and persists at very high vacuum.
Ramsay's 13-year-old son asked: What are you going to call the new gas? I should like to call it Novum.
Ramsay liked the suggestion but, wanting to maintain the chemical family's suffix -on,
called it Neon (Ne: #10). Neos (Greek) means new.
Ramsay and Travers acquired a new liquid-air machine that allowed production of larger amounts of Krypton and Neon. Repeated fractionation of Krypton resulted in their finding another gas with a beautiful blue spectra on July 12. They called this Xenon (Xe: #54). Xenos (Greek) means the stranger. They had discovered three members of the inert gas family within six weeks.
Marie Curie became interested in the source of radioactivity that Henri Becquerel discovered coming from pitchblende ore in 1897. Using a sensitive piezo-electric quartz electrometer invented by her husband, Pierre, Marie Curie surveyed known elements, finding that only two emitted appreciable ionizing radiation, Uranium and Thorium. Curie found that the amount of radiation depends exclusively on the quantity of the contained radioactive element but that pitchblende emits more radiation than its Uranium could alone cause. Seeking the cause of the additional radiation, Marie began chemical separations finding an intensely radioactive, trace component of pitchblende. In July 1898 Marie found a fraction of pitchblende containing Bismuth also contained a radioactive substance which they named Polonium. In December 1898 they found a Barium fraction contained another radioactive substance which they named Radium (Ra: #88). Radius (Latin) means ray. The Barium fraction containing Radium glowed in the dark! Moving to a Paris storage shed open to the weather, they spent three years isolating 0.1 g of Radium from a ton of scrap Uranium ore donated by the Austrian government. Wilhelm Ostwald described the laboratory as a cross between a horse-stable and a potato-cellar, and if I had not seen the worktable with the chemical apparatus, I would have thought it a practical joke.
Pierre and a student noticed a speck of Radium emits heat. This was the first hint of nuclear energy.
André Debierne was a young chemist who was among a handful of close friends that spent Sunday´s at the Curie home. In 1899 he found that adding ammonium hydroxide to a solution of dissolved pitchblende precipitated another radioactive element with the rare earths. Because of its radioactivity, he called it Actinium (Ac: #89). Aktinos (Greek) means ray. It was later found to produce a decay series that emits radiation similar to that of Radium.
While Curies viewed each source of radiation as a separate element, purified samples of Uranium and Radium were soon found to provide substances with differing intensities and half-lives. Before the nature of radiation was understood, before it was understood that an element can be transformed into a different element, and before the existence of isotopes was proposed, the discovery of these substances created massive confusion. In 1900 William Crookes found Uranium contained a substance soluble in ammonium hydroxide and ammonium carbonate he called Uranium X. Others found there were two Uraniums which they called Uranium 1 and Uranium 2. Uranium X1 formed Uranium X2. Uranium X1 was found to produce two kinds of beta rays resulting in Uranium X2 and Z. In 1911 Uranium Y was found. Eventually they were placed in a sequential series:
Uranium 1 → Uranium X1 → Uranium X2 → Uranium Z → Uranium 2 → Uranium Y.
A similar series was found to include Radium:
Ionium → Radium → radium emanation
 Curies noted that air in contact with Radium becomes radioactive. In 1900 Friedrich Ernst Dorn (b1848, d1916) showed that this is due to a gas produced from Radium. This nitron, or emanation, was eventually called Radon (Rn: #86). Recall that Radius is Latin for ray. The suffix -on was used to signify that it is a noble gas. Ernest Rutherford (b1871, d1937), physicist from New Zealand, and Frederick Soddy (b1877, d1956, ←at left), chemist from England, both teaching at McGill University in Canada, collaborated to investigate the radioactive gas emanation from Thorium discovered by Rutherford. After showing that high temperature had no effect on the rate of production, they cooled the substance and condensed liquid Radon. They proposed a theory of radioactivity in 1902 for which Soddy used the term transmutation (ala alchemy!) to describe the spontaneous disintegration of radioactive elements into new elements at a rate characteristic for each element. Soddy and Rutherford proposed the two decay series (which included those above), one starting with Uranium ending with Lead, and the other starting with Thorium (Ionium) and also ending in Lead. They realized that a large amount of energy is involved in radioactive decay, and Soddy thought there might be practical uses if a way could be found to rapidly release the energy.
Curies noted that air in contact with Radium becomes radioactive. In 1900 Friedrich Ernst Dorn (b1848, d1916) showed that this is due to a gas produced from Radium. This nitron, or emanation, was eventually called Radon (Rn: #86). Recall that Radius is Latin for ray. The suffix -on was used to signify that it is a noble gas. Ernest Rutherford (b1871, d1937), physicist from New Zealand, and Frederick Soddy (b1877, d1956, ←at left), chemist from England, both teaching at McGill University in Canada, collaborated to investigate the radioactive gas emanation from Thorium discovered by Rutherford. After showing that high temperature had no effect on the rate of production, they cooled the substance and condensed liquid Radon. They proposed a theory of radioactivity in 1902 for which Soddy used the term transmutation (ala alchemy!) to describe the spontaneous disintegration of radioactive elements into new elements at a rate characteristic for each element. Soddy and Rutherford proposed the two decay series (which included those above), one starting with Uranium ending with Lead, and the other starting with Thorium (Ionium) and also ending in Lead. They realized that a large amount of energy is involved in radioactive decay, and Soddy thought there might be practical uses if a way could be found to rapidly release the energy.
In 1903 Soddy left Canada to work with William Ramsay in London. Seeing Radium for sale in a store window, Soddy bought 20 mg and they found that pure Radium produces a Helium spectra. Soddy concluded that Helium came from alpha rays. In 1910 William Ramsay and Robert Whytlaw Gray measured the density of Radon, the heaviest member of the inert gases, establishing its atomic mass.
In 1913 Frederick Soddy, now at Glasgow, proposed that an element emitting an alpha particle is transmuted into the element two spaces to the left on the periodic table, whereas an element emitting a beta particle is transmuted into the element immediately to the right. Similar rules for beta decay were independently proposed by Kasimir Fajans, Alexander Russell and George de Hevesy. The rules provide a way to understand the decay series and led to Soddy´s proposal of isotopes to explain differing atomic weights for samples of the same element produced by different decay modes. For his contributions for understanding radioactive decay and proposing isotopes he was awarded the 1921 Nobel Prize for Chemistry. World War I and Soddy´s subsequent appointment to a position at Oxford with old, poorly equipped facilities stimied Soddy from further discoveries.
Primary Information Sources:
- Mary Elvira Weeks, Discovery of the Elements, Journal of Chemical Education, 1945
- Aaron J. Ihde, The Development of Modern Chemistry, Dover, 1964 & 1984
- Laylin K. James, Nobel Laureates in Chemistry 1901-1992, American Chemical Society & Chemical Heritage Foundation,1993
![]()

 The Greeks had proposed that the
The Greeks had proposed that the  The great Swedish chemist Jön Berzelius (←at left) became an ardent supporter of the new chemistry of Lavoisier and the subsequent atomic theory of John Dalton. Johan August Arfwedson (b1792, d1841) was a skillful young chemist studying chemistry under Berzelius. In 1817 he analyzed a rock previously discovered by a Brazilian, José Bonifácio de Andrada e Silva, while touring the Scandinavian island of Utö which is two miles into the Baltic. Several chemists had previously failed to investigate the red color this stone imparted to flames, or the puzzling losses during its analysis. Arfwedson found the rock was roughly 80% silica, 17% alumina, and 3% alkali. He found the alkali did not precipitate in tartaric acid like potassium and was not magnesium. Calculating its composition by presuming it to be soda resulted in a 5% excess. Two careful repetitions convinced Arfwedson that it was a new element which has a greater capacity to react than the other alkalies. Berzelius wrote to Berthollet:
The great Swedish chemist Jön Berzelius (←at left) became an ardent supporter of the new chemistry of Lavoisier and the subsequent atomic theory of John Dalton. Johan August Arfwedson (b1792, d1841) was a skillful young chemist studying chemistry under Berzelius. In 1817 he analyzed a rock previously discovered by a Brazilian, José Bonifácio de Andrada e Silva, while touring the Scandinavian island of Utö which is two miles into the Baltic. Several chemists had previously failed to investigate the red color this stone imparted to flames, or the puzzling losses during its analysis. Arfwedson found the rock was roughly 80% silica, 17% alumina, and 3% alkali. He found the alkali did not precipitate in tartaric acid like potassium and was not magnesium. Calculating its composition by presuming it to be soda resulted in a 5% excess. Two careful repetitions convinced Arfwedson that it was a new element which has a greater capacity to react than the other alkalies. Berzelius wrote to Berthollet:  Carl Gustav Mosander (b1797,d1858, at right→), an assistant of Berzelius, lived the house with Berzelius and his wife for years. Later he had an apartment adjoining the Stockholm mineral collections he tended. He also was professor and had charge of the chemical laboratory for medical students. Father Moses, as Berzelius and their German friend Wöhler called him, partially separated cerite with hot dilute nitric acid. He retained the old name
Carl Gustav Mosander (b1797,d1858, at right→), an assistant of Berzelius, lived the house with Berzelius and his wife for years. Later he had an apartment adjoining the Stockholm mineral collections he tended. He also was professor and had charge of the chemical laboratory for medical students. Father Moses, as Berzelius and their German friend Wöhler called him, partially separated cerite with hot dilute nitric acid. He retained the old name  Carl Auer was a quiet, industrious, unsociable boy from Austria who became a favorite student of Bunsen in Heidelberg. When he showed Bunsen a tiny handful of rare earth minerals he had collected from the north, Bunsen laughingly encouraged him to begin a study of them. Auer did so for the rest of his life. A number of chemists believed that Didymium was a mixture of elements but none had made a successful separation. In 1885 at age 26 Auer announced to the Vienna Academy of Sciences that by repeated fractionation of ammonium didymium nitrate, he had succeeded in splinting didymia into two earths. He proposed the names Praseodymium = Pr (#59): Praseios, (Greek) for leek-green since it has greenish salts, and didymos meaning twin and Neodymium = Nd (#60) Neos (Greek) means new.
Carl Auer was a quiet, industrious, unsociable boy from Austria who became a favorite student of Bunsen in Heidelberg. When he showed Bunsen a tiny handful of rare earth minerals he had collected from the north, Bunsen laughingly encouraged him to begin a study of them. Auer did so for the rest of his life. A number of chemists believed that Didymium was a mixture of elements but none had made a successful separation. In 1885 at age 26 Auer announced to the Vienna Academy of Sciences that by repeated fractionation of ammonium didymium nitrate, he had succeeded in splinting didymia into two earths. He proposed the names Praseodymium = Pr (#59): Praseios, (Greek) for leek-green since it has greenish salts, and didymos meaning twin and Neodymium = Nd (#60) Neos (Greek) means new. In general the lanthanide series was difficult to isolate because they are chemically nearly identical. They occur closely associated in very complex minerals. They have all been obtained by elaborate and laborious fractionations. As a result they are often called rare earths. While some are moderately abundant on earth, their pure elements are rare and costly even in the form of their earths (compounds). In 1886 Lecoq de Boisbaudran started with what was considered
In general the lanthanide series was difficult to isolate because they are chemically nearly identical. They occur closely associated in very complex minerals. They have all been obtained by elaborate and laborious fractionations. As a result they are often called rare earths. While some are moderately abundant on earth, their pure elements are rare and costly even in the form of their earths (compounds). In 1886 Lecoq de Boisbaudran started with what was considered  In 1785 Henry Cavendish thought there might be an unknown gas in the atmosphere when, after passing electric sparks through air, he found a small part of the phlogistonated air (later called Nitrogen) failed to oxidize. The residue was small, not more than 1/120 of the phlogistinated air, and was forgotten as trivial. A century later Robert John Strutt, third Lord Rayleigh, (b1842, d1919, at right→) began an investigation of gas densities. Known for his clear thinking, Lord Rayleigh succeeded upon Clerk Maxwell´s death to Professor at Cavendish Laboratory. He attracted increased enrollment and admitted women for the first time. Rayleigh found that Oxygen was NOT exactly 16 times heavier than Hydrogen, but 15.882. He was puzzled when Nitrogen from ammonia was slightly lighter than that from air. He thought of four possible explanations and was working to eliminate each.
In 1785 Henry Cavendish thought there might be an unknown gas in the atmosphere when, after passing electric sparks through air, he found a small part of the phlogistonated air (later called Nitrogen) failed to oxidize. The residue was small, not more than 1/120 of the phlogistinated air, and was forgotten as trivial. A century later Robert John Strutt, third Lord Rayleigh, (b1842, d1919, at right→) began an investigation of gas densities. Known for his clear thinking, Lord Rayleigh succeeded upon Clerk Maxwell´s death to Professor at Cavendish Laboratory. He attracted increased enrollment and admitted women for the first time. Rayleigh found that Oxygen was NOT exactly 16 times heavier than Hydrogen, but 15.882. He was puzzled when Nitrogen from ammonia was slightly lighter than that from air. He thought of four possible explanations and was working to eliminate each. William Ramsay (b1852, d1916, ←at left) was known to sit quietly as a child through long boring sermons; he spent that time learning to read French and German Bibles by comparing their text to the English he had memorized. Ramsay also worked out geometry propositions studying church window designs. He did advanced chemistry and glass blowing in his bedroom. He excelled in individual sports and drew crowds when diving. He gained permission from Lord Rayleigh to investigate atmospheric Nitrogen. He found after removing Nitrogen by hot Magnesium, 1/80 of the volume remained with a density of 19.086 g/cm3. Examining its spectrum, Ramsey noted groups of red and green lines not due to other gases. Exchanging daily letters with Rayleigh, Ramsey wrote on May 24, 1894:
William Ramsay (b1852, d1916, ←at left) was known to sit quietly as a child through long boring sermons; he spent that time learning to read French and German Bibles by comparing their text to the English he had memorized. Ramsay also worked out geometry propositions studying church window designs. He did advanced chemistry and glass blowing in his bedroom. He excelled in individual sports and drew crowds when diving. He gained permission from Lord Rayleigh to investigate atmospheric Nitrogen. He found after removing Nitrogen by hot Magnesium, 1/80 of the volume remained with a density of 19.086 g/cm3. Examining its spectrum, Ramsey noted groups of red and green lines not due to other gases. Exchanging daily letters with Rayleigh, Ramsey wrote on May 24, 1894:  Joined by an assistant, Morris William Travers (at right→), Ramsay continued to search for another member of the expected inert gas family lighter than Argon. They failed to find such a gas in rare minerals. Given a liter of liquid air, they used it to develop their skills manipulating gases. After most of the N2, O2, and Ar had distilled from liquefied air, they found the remains still contained oxygen and nitrogen which they removed with red-hot copper and magnesium. After lunch May 30, 1898, they observed the spectra of the inert remaining 25 cm3 of the original liter. Unique bright yellow and brilliant green lines suggested a new element which, because it remained hidden in the liquid, they named Krypton (Kr: #36). Kryptos (Greek) means hidden. Working until 11 PM Ramsay and Travers measured the density that placed it between Bromine and Rubidium on the periodic table. Since this was NOT the expected gas lighter than Argon, they continued their search.
Joined by an assistant, Morris William Travers (at right→), Ramsay continued to search for another member of the expected inert gas family lighter than Argon. They failed to find such a gas in rare minerals. Given a liter of liquid air, they used it to develop their skills manipulating gases. After most of the N2, O2, and Ar had distilled from liquefied air, they found the remains still contained oxygen and nitrogen which they removed with red-hot copper and magnesium. After lunch May 30, 1898, they observed the spectra of the inert remaining 25 cm3 of the original liter. Unique bright yellow and brilliant green lines suggested a new element which, because it remained hidden in the liquid, they named Krypton (Kr: #36). Kryptos (Greek) means hidden. Working until 11 PM Ramsay and Travers measured the density that placed it between Bromine and Rubidium on the periodic table. Since this was NOT the expected gas lighter than Argon, they continued their search. Curies noted that air in contact with Radium becomes radioactive. In 1900 Friedrich Ernst Dorn (b1848, d1916) showed that this is due to a gas produced from Radium. This nitron, or emanation, was eventually called Radon (Rn: #86). Recall that Radius is Latin for ray. The suffix -on was used to signify that it is a noble gas.
Curies noted that air in contact with Radium becomes radioactive. In 1900 Friedrich Ernst Dorn (b1848, d1916) showed that this is due to a gas produced from Radium. This nitron, or emanation, was eventually called Radon (Rn: #86). Recall that Radius is Latin for ray. The suffix -on was used to signify that it is a noble gas.