![]()
![]()
The same year, 1913, as Niels Bohr proposed a way to begin to understand atomic spectra and chemical bonding, Frederick Soddy proposed a way to understand radioactivity. In both areas there remained lots of questions. Hans Geiger and Hans Marsden working under Ernest Rutherford's guidance had discovered by 1911 evidence that most of an atom's mass resided in a very tiny nucleus. Lord Kelvin had proposed that such atomic nuclei might contain Helium nuclei and electrons to provide the particles needed for α (alpha) and β (beta) decay. But Soddy's proposal skirted around the issue and suggested that radioactive nuclei undergo transformations emitting Helium ions (α) or electrons (β). It had been proposed that atomic nuclei might be composed of positive particles (called protons equivalent to the nuclei of common Hydrogen atoms) plus enough electrons to result in a positive electric charge on the nucleus equal to its atomic number. While this proton-electron theory of atomic nuclei could explain charges and masses for α and β decay, it seemed inconsistent with Bohr's theory of the electrons. The smallest electron orbit (n = 1) permitted by Bohr was too large to be contained in a nucleus. And measurements of magnetic moments of atoms were inconsistent with the number of particles needed for this theory.
Yet recent precise measurements of most atomic weights were near integer values suggesting that atomic nuclei are composed of particles with masses of about one atomic mass unit. The magnetic moments of atoms were consistent with an alternate theory that nuclei are composed of protons and similar massed neutral particles which in 1920 Ernest Rutherford proposed and named the neutron. While such particles were commonly believed to exist, neither protons nor neutrons had been actually verified.
Meanwhile others tried to determine if elements with non-integer atomic weights were actually a mixture of isotopes, each with an atomic weight that fit the theory of nuclei composed of particles with uniform mass. J.J.Thomson (1856-1940), A.J.Dempster (1886-1950) and others extended Thomson's procedure for measuring charge to mass ratios. By ionizing Neon gas, accelerating the Neon ions by electric field to a known velocity, and measuring their curved paths in a uniform magnetic field, Thomson was able to demonstrate that Neon, atomic weight 20.15, was indeed composed of two isotopes, one 2010Ne and a lesser amount of 2210Ne. Francis William Aston (1877-1945), working with Thomson, developed this process of weighing isotopes which became known as mass spectrometry. (Read Aston's report about integer atomic weights.) Aston further devised a way to gradually separate isotopes of an element by passing them through numerous porous walls using diffusion. (According the Kinetic Molecular Theory, at any particular temperature, lighter atoms move faster on average so typically cross the porous barrier slightly sooner. Aston was awarded the 1922 Nobel Prize in chemistry for accurate isotope mass determinations.) His gaseous diffusion process was later used during WWII to separate Uranium isotopes for the first atomic bombs.
 In 1919 Rutherford noted that when α particles from Bismuth-214 travelled through the air, occasionally one of the particles travelled much further than was typical for α particles. Generally the length of an α track in a cloud chamber depends on the particle's energy. α tracks are never more than a few centimeters long. So Rutherford guessed that an α had collided with a nitrogen atom in the air which underwent an artificial radioactive transformation releasing a less charged, more penetrating particle matching the expected properties of a proton (the nucleus of a Hydrogen, 11H). (Read Rutherford's report.) Careful measurements confirmed Rutherford's discovery. In 1925 P.M.S.Blackett photographed α particle tracks in a cloud chamber confirming that the α was actually captured by the Nitrogen rather than chipping off a proton then continuing onward. (Note in Blackett's photo that at the red arrow an α travelling to the right strikes a Nitrogen. The proton leaves the long track upwards and the newly formed Oxygen recoils slightly downward to the right. But there is no sign of the α continuing onward.):
In 1919 Rutherford noted that when α particles from Bismuth-214 travelled through the air, occasionally one of the particles travelled much further than was typical for α particles. Generally the length of an α track in a cloud chamber depends on the particle's energy. α tracks are never more than a few centimeters long. So Rutherford guessed that an α had collided with a nitrogen atom in the air which underwent an artificial radioactive transformation releasing a less charged, more penetrating particle matching the expected properties of a proton (the nucleus of a Hydrogen, 11H). (Read Rutherford's report.) Careful measurements confirmed Rutherford's discovery. In 1925 P.M.S.Blackett photographed α particle tracks in a cloud chamber confirming that the α was actually captured by the Nitrogen rather than chipping off a proton then continuing onward. (Note in Blackett's photo that at the red arrow an α travelling to the right strikes a Nitrogen. The proton leaves the long track upwards and the newly formed Oxygen recoils slightly downward to the right. But there is no sign of the α continuing onward.):
Rutherford had discovered the long anticipated proton. And Rutherford had discovered that on rare occasions (only one transformation for every million α), collision with an α particle can cause a nuclear transformation that would not otherwise have occurred.
Between 1921 and 1924 Rutherford and James Chadwick found that they could produce artificial transformation of all light elements from Boron (#5) to Potassium (#19) except for Carbon (#6) and Oxygen (#8).
The existence of the neutron was more elusive because there was no apparent natural source of free neutrons and all known detectors of radiation depended on the radiation either carrying electric charge or causing the emission of electric charges which could be subsequently detected. So with the neutron anticipated to be nearly undetectable, physicists investigated other challenges.
W.G.Bothe and H.Becker, working in 1930 in Germany, discovered that when samples of Beryllium (#4) or Boron were struck by α particles, they emitted γ rays. (These gamma rays are electromagnetic waves like X-rays except with even higher energies.) Neither X-rays nor γ rays are electrically charged so they do not leave trails in photographic film or cloud chambers. But when they are absorbed by matter, the large amount of energy causes the disintegration of one or more atoms creating a burst of charged particles what can be detected. A number of investigators measured the penetration of these γ rays in Lead finding their energy to be about 10 MeV, an energy larger than carried by any previously discovered γ rays. Frédéric Joliot and Irène Curie (daughter of Marie) in Paris measured the absorption of these γ rays in paraffin (composed of Hydrogen and Carbon in roughly a 2:1 ratio). Expecting the absorbed energy to eject protons, 11H, they noted a larger number of ejected protons than expected. The emerging protons carried about 5 MeV of energy, an amount also much greater than expected.
 If γ rays conserved energy and momentum as found by Arthur Compton for X-rays, the γ rays should have at least 50 MeV of energy in order to transfer 5 MeV to the protons. While some physicists pondered if these observations would lead to additional restraints on classical physics much like Bohr's theory required inside the atom, James Chadwick (1891-1974) proposed a classical solution He had earlier noted similarly perplexing results for recoil nuclei from other light atoms including Helium (#2), Lithium (#3), Carbon (#6), Nitrogen (#7), and Argon (#18). In a February 1932 letter to the Editor of Nature, Chadwick proposed radiation from the Beryllium, which left no tracks in cloud chambers just like γ rays, was NOT γ rays but rather the elusive neutron. The neutrons would be created by the following nuclear transformation:
If γ rays conserved energy and momentum as found by Arthur Compton for X-rays, the γ rays should have at least 50 MeV of energy in order to transfer 5 MeV to the protons. While some physicists pondered if these observations would lead to additional restraints on classical physics much like Bohr's theory required inside the atom, James Chadwick (1891-1974) proposed a classical solution He had earlier noted similarly perplexing results for recoil nuclei from other light atoms including Helium (#2), Lithium (#3), Carbon (#6), Nitrogen (#7), and Argon (#18). In a February 1932 letter to the Editor of Nature, Chadwick proposed radiation from the Beryllium, which left no tracks in cloud chambers just like γ rays, was NOT γ rays but rather the elusive neutron. The neutrons would be created by the following nuclear transformation:
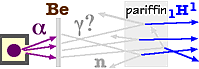 Being about the same mass as a proton, the 10 MeV neutron in a typical subsequent collision in the paraffin would recoil, transferring about half its energy to the proton being ejected, giving each proton about 5 MeV of energy.
Being about the same mass as a proton, the 10 MeV neutron in a typical subsequent collision in the paraffin would recoil, transferring about half its energy to the proton being ejected, giving each proton about 5 MeV of energy.
Almost immediately Enrico Fermi proposed to his small research group in Italy that neutrons, lacking any electric charge, should provide a better tool for causing nuclear transformations. While α particles had a positive charge and were repelled as they approached a nucleus, there were no known repulsive forces to prevent even slow neutrons from being absorbed by a nucleus.
Our senses are nearly blind to high energy radiation at low intensities. By two serendipitous accidents radioactivity was discovered. Wilhelm Konrad Röntgen (1845-1923) noticed on November 8, 1895 that a scrap of paper coated with barium platinocyanide fluoresced nearby an evacuated glass tube enclosing a high voltage spark. Then less than four months later Antoine Henri Becquerel (1852-1908), deterred by poor weather discovered that, even when not excited by any apparent source of energy, a mineral called pitchblende emits similar penetrating radiation. (Review their discoveries.)
But neither the glow of florescent minerals, the exposure of photographic plates or Curies timed electric discharge provided much view of the paths of radiation.

Between 1894 and 1912Charles Thomson Rees Wilson, (1869-1959 at right) devised a cloud chamber first to study weather, but which he found also allowed radiation carrying electric charge to leave contrails much as high flying aircraft occasionally leave trails of moisture in saturated air. Air nearly saturated with water would be cooled by a rapidly expanding piston, making the air ripe for cloud formation. Ionized air molecules left in the wake of passing charged radiation would provide nuclei for droplet formation. As the droplets grew preferentially along the radiation's path, light scattered off the line of droplets would reveal the path followed by the radiation.
 In the presence of a perpendicular magnetic field, the charged particles would follow curved paths which could provide information about their mass. Recall the right hand rule that a moving positively charged (q) particle will be deflected (force F) perpendicular to both the magnetic field B and the particle's velocity v:
In the presence of a perpendicular magnetic field, the charged particles would follow curved paths which could provide information about their mass. Recall the right hand rule that a moving positively charged (q) particle will be deflected (force F) perpendicular to both the magnetic field B and the particle's velocity v:
Negative particles will be deflected in the opposite direction. and uncharged particles will move in undeflected straight lines (q = 0 gives F = 0).
While cloud chambers of condensing vapor have been retired and replaced by faster detectors, archived photographs from such chambers still provide visual insights into the nature of high energy particles and the procedures we still used to learn about the particles' properties and interactions. Today such trails are electronically detected then presented on computer screens looking much like the older photographs. So understanding the imaging processes and studying the particle tracks can help us understand the nature of matter.
In this investigation we will visit the web site at the Cavendish Laboratory at the University of Cambridge where Wilson performed his experiments, view their animations, then try to interpret a historical cloud chamber photograph.
In Lab VI-6 we will use a computer simulator at Stanford University to calculate in real time possible tracks for high energy collisions of our choosing. In Lab VII-3 we will view, compare and interpret more real tracks on photographs archived by CERN, the European high energy research institution.
![]()
next Experiment
to ie-Physics menu
to site menu