![]()
![]()
In 1897 J.J.Thomson had deflected cathode ray particles using magnetic fields to determine the ratio of the electric charge to the mass of the particles. The ratio about two thousand times larger than the ratio for the lightest chemical ion, hydrogen = H+1, was the basis for announcing the discovery of what became know as electrons. If the charges were comparable, the electron mass would be roughly 1/2000 that of the lightest atom.
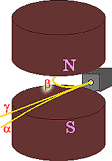 During 1900 the Curies collected beta radiation, β, in an electroscope gathering evidence that beta rays, like electrons, are also composed of negatively charged particles. When a beam of mixed forms of radiation was allowed to emerge from a hole in a lead container, the beta rays, β, were deflected one way in a magnetic field (as shown to left) while the alpha rays, α, were deflected the opposite direction. (Gamma rays, γ, were undeflected.) Thus the alpha rays were known to carry positive electric charge. Using the same procedure as previously used by J.J.Thomson, Becquerel confirmed the charge to mass ratio for beta rays matched that of electrons. Beta must be electrons emerging at high speeds from some radioactive materials.
During 1900 the Curies collected beta radiation, β, in an electroscope gathering evidence that beta rays, like electrons, are also composed of negatively charged particles. When a beam of mixed forms of radiation was allowed to emerge from a hole in a lead container, the beta rays, β, were deflected one way in a magnetic field (as shown to left) while the alpha rays, α, were deflected the opposite direction. (Gamma rays, γ, were undeflected.) Thus the alpha rays were known to carry positive electric charge. Using the same procedure as previously used by J.J.Thomson, Becquerel confirmed the charge to mass ratio for beta rays matched that of electrons. Beta must be electrons emerging at high speeds from some radioactive materials.
 Measuring the charge to mass ratio for alpha rays required a stronger magnetic field because that ratio is about a factor of 4000 smaller. If the electric charges are comparable, the mass might be roughly double that of a hydrogen ion. In 1903 Sir William Ramsey and Frederick Soddy discovered helium imprisoned in radioactive minerals. Ernest Rutherford proposed that alpha particles could be Helium ions, He2+, since they also would have the correct charge to mass ratio. But it took most of the rest of the decade to confirm.
Measuring the charge to mass ratio for alpha rays required a stronger magnetic field because that ratio is about a factor of 4000 smaller. If the electric charges are comparable, the mass might be roughly double that of a hydrogen ion. In 1903 Sir William Ramsey and Frederick Soddy discovered helium imprisoned in radioactive minerals. Ernest Rutherford proposed that alpha particles could be Helium ions, He2+, since they also would have the correct charge to mass ratio. But it took most of the rest of the decade to confirm.
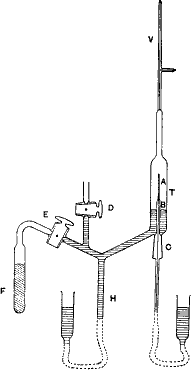
T.D.Royds and Ernest Rutherford placed in an inner glass container with extremely thin walls (A in their diagram) Radon gas (then called Radium emanation) known to emit alpha particles. Alpha radiation escaped into the evacuated outer glass container (T) where the ions picked up electrons with collisions with the glass. The outer container was flooded after 2, 4, and 6 days with Mercury compressing any new gas into the very narrow neck (V in their diagram). There a spark excited any atoms to produce a visible spectra. Each time the spectra was checked, the well known spectra of Helium grew brighter confirming that alpha radiation is composed of Helium ions. (Read their original report.)
 During the 19th Century chemists began to see the value of understanding materials as composed of atoms. While atoms seemed the only explanation for some properties such as the constant mass ratios for each compound, there were many who doubted that atoms really exist. As the 20th Century began, evidence such as Brownian motion as mathematically explained by Albert Einstein helped convince most of the remaining doubters. Each kind of chemical element was thought to be composed of identical atoms which are indestructible and unchangeable. But emission of radiation, Helium ions and the much smaller beta, seemed inconsistent with this atomic theory. It was also unclear how radioactive atoms could possibly release large amounts of energy nearly undiminished over time. While there were still empty positions in the otherwise very successful periodic chart of the elements, the recent discovery of a large number of radioactive elements seemed to defy placement. Many of these new elements had nearly identical chemical properties but emitted different kinds of radiation at different rates.
During the 19th Century chemists began to see the value of understanding materials as composed of atoms. While atoms seemed the only explanation for some properties such as the constant mass ratios for each compound, there were many who doubted that atoms really exist. As the 20th Century began, evidence such as Brownian motion as mathematically explained by Albert Einstein helped convince most of the remaining doubters. Each kind of chemical element was thought to be composed of identical atoms which are indestructible and unchangeable. But emission of radiation, Helium ions and the much smaller beta, seemed inconsistent with this atomic theory. It was also unclear how radioactive atoms could possibly release large amounts of energy nearly undiminished over time. While there were still empty positions in the otherwise very successful periodic chart of the elements, the recent discovery of a large number of radioactive elements seemed to defy placement. Many of these new elements had nearly identical chemical properties but emitted different kinds of radiation at different rates.
 The solutions were provided in 1913 by a bold theory of radioactive transformations proposed independently by Frederick Soddy (1877-1956, photo at left), in England (see Soddy papers 1, 2, 3) and Kasimir Fajans (1887-1975) in Germany (see Fajans' paper). Lord Kelvin had proposed that Radium was some sort of compound of Lead and Helium. Instead Soddy proposed that several forms of an element could exist, which he called isotopes, which would be identical in most properties but differ in atomic mass and radioactivity. (For this idea Soddy won the 1921 Nobel Prize in chemistry.) According th Soddy and Fajans, an isotope emitting an alpha particle would result in a transformation to an atom two chemical families to the left and decreased by four mass units. An isotope emitting a beta particle would transform to an atom in the next chemical family to the right but retain its prior mass.
The solutions were provided in 1913 by a bold theory of radioactive transformations proposed independently by Frederick Soddy (1877-1956, photo at left), in England (see Soddy papers 1, 2, 3) and Kasimir Fajans (1887-1975) in Germany (see Fajans' paper). Lord Kelvin had proposed that Radium was some sort of compound of Lead and Helium. Instead Soddy proposed that several forms of an element could exist, which he called isotopes, which would be identical in most properties but differ in atomic mass and radioactivity. (For this idea Soddy won the 1921 Nobel Prize in chemistry.) According th Soddy and Fajans, an isotope emitting an alpha particle would result in a transformation to an atom two chemical families to the left and decreased by four mass units. An isotope emitting a beta particle would transform to an atom in the next chemical family to the right but retain its prior mass.
Teams of chemists and physicists worked to unravelling the confusion. After Pierre Curie's untimely death, Marie continued her investigations in Paris, interrupted by WWI. She was in charge of 37 researchers by 1931. Ernest Rutherford started research in Montreal, Canada, moved to Manchester, England, and finished his career directing research at Cambridge. Otto Hahn, coming from study with Rutherford at McGill University in Montreal and Lise Mietner established research on radioactive materials in Berlin. Mietner had come from Vienna where there were others interested in radiation. A bit later Enrico Fermi formed a similar research group in Rome.
Stefanie Horovitz (1887-1940) in Vienna determined the atomic weight of lead from pitchblende was 206.736 compared to 207.190 for normal
lead, a clear violation of the claim that all atoms of an element are identical. (She and her sister were liquidated by the Nazis in 1940.)
According the Soddy, atoms such as these two isotopes (Greek: isos = same & topos = place) of lead should occupy the same place on the periodic chart even though they likely have different radiation properties.
Research often involved tedious chemical separations of elements followed by measurements of radiation intensities (the distance travelled or ease of absorption) and half lives (time needed for intensities to decrease in half). By identifying the type of radiation and using Soddy's rules the element produced could be predicted. Determination of atomic weight helped placement in the periodic chart and helped confirm a radioactive series of events.
It is now customary to indicate specify a particular isotope by using exponents and subscripts. 20782Pb represents lead (symbol Pb = Plumbum), atomic number 82, with mass of 207.
meta-stablefor having longer lifetimes.)
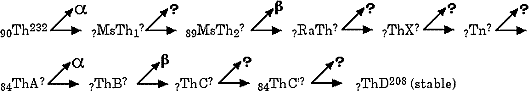
Not understanding the potential effects of radiation on living cells, many researchers took no precautions against radiation. Pierre Curie carried a warm, glowing sample of Radium around in his pocket to show people. Marie Curie kept a glowing sample as a night light beside her bed. Only after early researchers became ill did they begin to realize the need for safety precautions. Marie Curie helped form an international committee to establish standards and safety regulations. Still most believed that fresh air and time away from their researches provided adequate recovery. It wasn't until much later when many early investigators reached retirement ages that the connection between radiation exposures and various forms of cancer became apparent.
![]()
next Experiment
to ie-Physics menu
to site menu