![]()
![]()
It has been long known that various substances distinctly color flames. In common temperature flames, the energy excites the metals causing them to glow with distinct colors. In higher temperature flames, non-metals will also emit distinct colors.
 BaCl2 |
 CuCl2 |
 KCl |
 LiCl |
 NaCl |
People have probably always noticed the colors of rainbows which result when light reflected inside a nearly round raindrop leaves the drop at a surface not parallel to where it entered. Isaac Newton was the first to record experiments where he used two prisms, the first to separate sunlight into a rainbow of colors, and the second to reassemble it back to nearly white light. Newton noted that when he blocked some colors where they were separated between the prisms, the second prism was unable to reconstruct white sunlight, but instead formed some colored light. Newton concluded that all the colors of the rainbow were needed to make white light.
In the 1850s physicist Gustav Kirchhoff (in Königsberg, Prussia ←below left) and chemist Robert Bunsen (in Gottingen, Germany, below right→) invented a spectroscope which separated the colors of light so they could systematically study light generated by chemicals excited in flames. In 1859, they reported that they had found that each chemical element emitted unique spectral colors which were not effected by the presence of other elements. Such spectral colors provided a new tool for identifying even trace ingredients in unknown composition materials. Using this technique, Robert Bunsen found two new elements, one he named Cesium for its blue spectral line, and the second named Rubidium for its red spectral line.
Another method they used to create spectra was to bombard a gas with a high voltage electric spark. It was also noted that a kind of negative spectra
could also be obtained by passing white light through a container of room temperature gas. Kirchhoff described the relationship between three types of spectra as:


 |
| as recorded by two different (imperfect) cameras |
 Hans Geiger and Hans Marsden, working in Ernest Rutherford's lab, unexpectedly found alpha radiation was occasionally reflected from a Gold target. Rutherford deduced that this required a tiny but massive atomic core which he named the nucleus. They found that each atom has a nucleus containing nearly all the atom's mass and a positive electric charge equal the atomic number. This created a dilemma. If an atom's negative electrons were stationed in the remaining atomic space, the electrical attraction would pull the electrons toward the nucleus, collapsing the atom. Alternatively, if the electrons were placed in motion orbiting the nucleus, the Maxwell's electromagnetic laws governing accelerated electric charges would require that each electron broadcast away its energy as electromagnetic waves resulting in a spiral crash into the nucleus. Neither stationary nor moving electrons could form stable atoms. That seemed to eliminate ALL possibilities!
Hans Geiger and Hans Marsden, working in Ernest Rutherford's lab, unexpectedly found alpha radiation was occasionally reflected from a Gold target. Rutherford deduced that this required a tiny but massive atomic core which he named the nucleus. They found that each atom has a nucleus containing nearly all the atom's mass and a positive electric charge equal the atomic number. This created a dilemma. If an atom's negative electrons were stationed in the remaining atomic space, the electrical attraction would pull the electrons toward the nucleus, collapsing the atom. Alternatively, if the electrons were placed in motion orbiting the nucleus, the Maxwell's electromagnetic laws governing accelerated electric charges would require that each electron broadcast away its energy as electromagnetic waves resulting in a spiral crash into the nucleus. Neither stationary nor moving electrons could form stable atoms. That seemed to eliminate ALL possibilities!
Niels Bohr (b1885 in Copenhagen, Denmark, d1962) ←at left) had studied at Cambridge University under J.J. Thomson (who discovered the electron) and Rutherford. In 1913 Bohr proposed that electrons did orbit the nucleus but that the electromagnetic laws somehow acted differently inside atoms. Bohr used Max Planck and Albert Einstein's radical quantum theory of energy by suggesting that the momenta of electron orbits inside atoms must be restricted to multiples of Planck's constant. Bohr proposed that since Planck's constant had the units of angular momentum, perhaps the angular momentum of each electron was specified by an integer (quanta = bundle) multiplied of Planck's constant. Since electrons would not be permitted to have fractional quanta, an electron could not gradually broadcast.
To have a stable orbit the centripetal force (on left) must be provided by the electrical force (on right)
mv2 / r = kqeqn / r2
where m & v are the electron's mass & velocity, r = orbit radius, q = nucleus & electron's charges, and k =
Coulomb's constant.
This simplifies to
mvr = kqeqn / v.
In this form Bohr recognized the left side is the angular momentum of the orbiting electron.
But Planck's constant, h, the heart of quantized energy, has units of angular momentum.
So Bohr set this equal integer, n, times Planck's constant; 2π (orbit is a circle):
mvr = nh / 2π
The total energy (potential + kinetic) for a particular (nth) orbit is
En = -kqeqn / 2r = -k2 2π2 mqeqn / n2h2
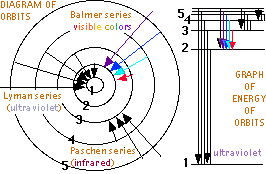 But Bohr proposed that an electron could absorb the correctly sized quanta of energy to be excited to any higher orbit allowed by a larger integer. Then the excited electron might spontaneously returning to a lower orbit, emitting the discrete color of light specified by the difference in energy of the two electron orbits. Bohr calculated the orbits possible, the energies a electron would have in each orbit, and the spectra colors for each orbit transition in the simplest atom, hydrogen. He found a precise match with the energy of the dozens of spectra lines of hydrogen known as the Lyman, Balmer, Paschen, Brackett and Pfund series.
But Bohr proposed that an electron could absorb the correctly sized quanta of energy to be excited to any higher orbit allowed by a larger integer. Then the excited electron might spontaneously returning to a lower orbit, emitting the discrete color of light specified by the difference in energy of the two electron orbits. Bohr calculated the orbits possible, the energies a electron would have in each orbit, and the spectra colors for each orbit transition in the simplest atom, hydrogen. He found a precise match with the energy of the dozens of spectra lines of hydrogen known as the Lyman, Balmer, Paschen, Brackett and Pfund series.
Noting that the periodic table has inert elements periodically, with elements that easily lose electrons on one side, and atoms that take electrons on the other side, Bohr proposed that filling or emptying an orbit that holds a specified maximum number of electrons. This finally provided the first explanation for the arrangement of the periodic table of the chemical elements! This proposal also made it possible to predict where other missing elements might be discovered.
| 0 | I | II | III | IV | V | VI | VII | VIII |
| He | Li | Be | B | C | N | O | F | |
| Ne | Na | Mg | Al | Si | P | S | Cl | |
| A | K | Ca | Sc | Ti | V | Cr | Mn | Fe Co Ni |
| Cu | Zn | Ga | Ge | As | Se | Br | ||
| Kr | Rb | Sr | Y | Zr | Nb | Mo | ? | Ru Rh Pd |
| Ag | Cd | In | Sn | Sb | Te | I | ||
| X | Cs | Ba | La | Ce | - | - | - | |
| - | - | Yb | - | Ta | W | - | Os Ir Pt | |
| Au | Hg | Tl | Pb | Bi | - | - | ||
| - | Ra | - | Th | - | U |
G.N. Lewis, Walther Kossel, and Irwing Langmuir in 1916 used the ideas of Bohr's theory to develop a new theory of chemical bonding related to full electron shells. Ions in polar materials such as salts gain or lose electrons so that the outer electron shell is full. Ions then bond by electrical attraction. Non-polar molecules such as in organic molecules share electrons in a covalent bond that fills the outer electron shells. Orbits may contain a limited number of electrons. Inert gases are atoms with full electron orbits.
 But Bohr's theory predicted incorrect spectra of atoms with more than one electron. His efforts to fix the theory failed.
But Bohr's theory predicted incorrect spectra of atoms with more than one electron. His efforts to fix the theory failed.
He returned to the University of Copenhagen to work on radioactive decay. For his work in atomic structure and radiation he received the 1922 Nobel prize in physics. (←Photo to left shows Bohr with grand kids in front of the house purchased with his Nobel prize award.)
Eventually Schrödinger and Heisenberg developed Quantum Wave Mechanics to replace Bohr's atomic theory. Working at the Niels Bohr Institute established by the Danish government, Bohr continued to be a leader and father-like organizer in the search for understanding of atoms and nuclei. Bohr fled Denmark during the Nazis occupation, carrying the announcement of fission from Meitner to the United States. He assisted the resulting Allied development of the atomic bomb. After the war he explained the need for international control of atomic weapons to Washington before returning to Denmark.
In August of 1997 the International Union of Pure and Applied Chemistry announced the official naming of element #107 as Bohrium (Bh).
(eventually to follow)
![]()
next Experiment
to ie-Physics menu
to site menu