


In 1900 Max Planck (born 1858, d1947, shown at right→) was attempting to develop a mathematical equation to accurately describe the energy emitted from a black body, an ideal emitter of electromagnetic radiation (i.e., a perfect light source. A heated kiln which emits energy through a small hole is a close approximation of a black body). Encountering difficulties, Planck tried a calculus trick of considering the energy to be emitted in small discrete amounts. Anticipated the size of these small energy lumps would be irrelevant and not remain in the final equation, Planck realized the radical implications when he found the size of the energy lumps remained in the final equation and thus is not irrelevant. The lump size is proportional the frequency of the radiation times a tiny number now called Planck's constant = 6.6 x 10-34 Joule-seconds. Planck thought the lumpy (now called quantum) emission of light energy might be due to the lumpy nature of atomic matter.
In 1905 Albert Einstein (b1879, d1955) recognized that Planck's constant in another equation describing the photoelectric effect, E = hν – W, implies that the term hν
requires light by its nature to be quantized. It is an inherent property of the energy of light, and not a consequence of matter being atomic.
 Rutherford created a dilemma when he proposed that each atom has a nucleus containing nearly all the atom's mass and a positive charge equal the atomic number. If an atom's negative electrons were stationed in the remaining atomic space, the electrical attraction would pull the electrons toward the nucleus, collapsing the atom. Alternatively, if the electrons were placed in motion orbiting the nucleus, the electromagnetic laws governing accelerated electric charges would require that each electron broadcast away its energy resulting in a spiral crash into the nucleus. Neither stationary nor moving electrons could form stable atoms. In 1913, Niels Bohr (b188, d1962, ←at left), after working with Rutherford, proposed that electrons did orbit the nucleus but that the electromagnetic laws somehow acted differently inside atoms. Bohr proposed that since Planck's constant had the units of angular momentum, perhaps the angular momentum of each electron is specified by an integer (quanta) multiplied by Planck's constant. Since electrons would not be permitted to have fractional quanta, an electron could not gradually broadcast.
Rutherford created a dilemma when he proposed that each atom has a nucleus containing nearly all the atom's mass and a positive charge equal the atomic number. If an atom's negative electrons were stationed in the remaining atomic space, the electrical attraction would pull the electrons toward the nucleus, collapsing the atom. Alternatively, if the electrons were placed in motion orbiting the nucleus, the electromagnetic laws governing accelerated electric charges would require that each electron broadcast away its energy resulting in a spiral crash into the nucleus. Neither stationary nor moving electrons could form stable atoms. In 1913, Niels Bohr (b188, d1962, ←at left), after working with Rutherford, proposed that electrons did orbit the nucleus but that the electromagnetic laws somehow acted differently inside atoms. Bohr proposed that since Planck's constant had the units of angular momentum, perhaps the angular momentum of each electron is specified by an integer (quanta) multiplied by Planck's constant. Since electrons would not be permitted to have fractional quanta, an electron could not gradually broadcast.
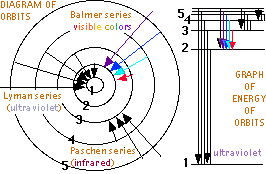
But Bohr proposed that an electron could absorb the correctly sized quanta of energy to be excited to any higher orbit allowed by a larger integer. Then the excited electron might spontaneously returning to a lower orbit (diagram near right→), emitting the discrete color of light specified by the difference in energy of the two electron orbits. Bohr calculated the orbits possible, the energies a electron would have in each orbit (graphed far right→), and the spectra colors for each orbit transition in the simplest atom, Hydrogen. He found a perfect match with each of the series of Hydrogen spectra: the four visible spectra lines (shown below↓) described by empirical formula devised by Johann Jakob Balmer (b1825, d1898) in 1885, a similar set of ultraviolet spectral lines from electron jumps to the first orbit discovered between 1906 and 1914 by Theodore Lyman (b1874, d1954), and infrared spectra from electrons jumping to the 3rd orbit discovered by Friedrich Paschen (b1865, d1947) in 1908. Bohr's calculations also correctly predicted additional spectra from electrons jumping to the 4th orbit and first detected by Frederick Sumner Brackett (b1896, d1988) in 1922, lines for the 5th orbit first detected by August Herman Pfund (b1879, d1949) in 1924 and lines to the 6th orbit detected by Curtis J. Humphreys (b1898, d1986) in 1953.
![]()
Noting that the periodic table has inert elements periodically, with elements that easily lose electrons on one side, and atoms that take electrons on the other side, Bohr proposed that filling or emptying an orbit that holds a specified maximum number of electrons could explain the table. This proposal made it possible to predict what other missing elements might be discovered.
| 0 | I | II | III | IV | V | VI | VII | VIII |
| He | Li | Be | B | C | N | O | F | |
| Ne | Na | Mg | Al | Si | P | S | Cl | |
| A | K | Ca | Sc | Ti | V | Cr | Mn | Fe Co Ni |
| Cu | Zn | Ga | Ge | As | Se | Br | ||
| Kr | Rb | Sr | Y | Zr | Nb | Mo | ? | Ru Rh Pd |
| Ag | Cd | In | Sn | Sb | Te | I | ||
| X | Cs | Ba | La | Ce | - | - | - | |
| - | - | Yb | - | Ta | W | - | Os Ir Pt | |
| Au | Hg | Tl | Pb | Bi | - | - | ||
| - | Ra | - | Th | - | U |


G.N. Lewis (b1875, d1946 in his laboratory at Berkeley, shown at right→), Walther Kossel (b1888 in Berlin, d1956 in Tübingen), and Irving Langmuir (b1881 in Brooklyn, d1957 at Woods Hole, MA, ← shown at left) in 1916 developed a new theory of chemical bonding related to full electron shells (i.e., orbits). Ions in polar materials such as salts gain or lose electrons so that the outer electron shell is full. Ions then bond by electrical attraction. Non-polar molecules such as in organic molecules share electrons in a covalent bond that fills the outer electron shells. Orbits may contain a limited number of electrons. Inert gases are atoms with filled electron orbits.


| introduction | Greeks | alchemy | Lavoisier | Dalton | Berzelius | molecules | spectra | electron | radiation | ↑ | isotopes | synthesis |
| to site menu | Discovery and Naming of Chemical Elements |
chemistry | physics | |||||||||
| created 23 March 2002 latest revision 4 May 2010 |
by D Trapp | |||||||||||