![]()
![]()
Earlier investigations repeated mentioned the need for energy to power various life processes. Living things do no create energy; energy is conserved and cannot be created or destroyed. Since energy, like most other things, can spontaneously spread out or be shared with its surroundings, the trick is for living organisms to either capture it as is passes by or to harvest energy from a source of concentrated potential energy. Plants capture light energy spreading out from the Sun and animals eat food with concentrated chemical energy. The following describes some of the details how we and other organisms utilize the energy in food.
We'll first look and how human metabolism works, and later compare and contrast that of other organisms. Humans are omnivores with different foods needing different chemical processes. The metabolism of carbohydrates, the most plentiful of our food sources, will be described first. That will be followed by description of how other foods use different initial processes, but later share a common energy extraction.
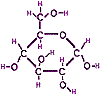 Carbohydrates have molecular formulas which seem to be a combination of equal or nearly equal amounts of Carbon (C) and water (H2O) such as glucose, C6H12O6. But the structural formulas (← glucose at left) show a Carbon chain with an abundance of hydroxyl (C-O-H) with occasional carbonyl (C=O) functional groups. These usually prefer a configuration and self-reaction which form five or six atom rings involving the carbonyl Oxygen (as C-O-C) with a handful of Carbons. These rings called monosaccharides can combined in pairs (disaccharides such as the table sugar, sucrose) or longer chains (polysaccharides such as glycogen, starches, cellulose, and gums). Plants make primarily glucose via photosynthesis but can convert it to other carbohydrates to meet the plants' needs.
Carbohydrates have molecular formulas which seem to be a combination of equal or nearly equal amounts of Carbon (C) and water (H2O) such as glucose, C6H12O6. But the structural formulas (← glucose at left) show a Carbon chain with an abundance of hydroxyl (C-O-H) with occasional carbonyl (C=O) functional groups. These usually prefer a configuration and self-reaction which form five or six atom rings involving the carbonyl Oxygen (as C-O-C) with a handful of Carbons. These rings called monosaccharides can combined in pairs (disaccharides such as the table sugar, sucrose) or longer chains (polysaccharides such as glycogen, starches, cellulose, and gums). Plants make primarily glucose via photosynthesis but can convert it to other carbohydrates to meet the plants' needs.
We eat a variety of carbohydrates. Taste buds in our tongue can detect the pleasant taste of many mono- and disaccharides providing encouragement to eat them. Amylase, an enzyme in our saliva digests a little of the tasteless starch to sweet disaccharide to encourage the eating of that source of potential energy. Additional amylase and maltase and other enzymes in the small intestine further digest the disaccharides and some polysaccarides to monosaccharides so the molecules are small enough to be absorbed into the blood stream. Some polysaccharides such as cellulose are not digestible by humans and so eventually pass out of the digestion track. The absorbed monosaccharides can be briefly stored as the polymer glycogen primarily by the liver under the direction of the hormone insulin. Long term energy storage is possible by converting to the lipid known as body fat. Generally monosaccharides are absorbed through protein gateways into individual cells which extract the energy in three assembly line processes collectively called metabolism. Overall the net reaction is
C6H12O6 + 6 O2 → 6 CO2 + 6 H2O + energy
 But that reaction in a single step would do little more than generate unusable heat energy. The world's best heat engines capture at best roughly a third of the energy into usable work and require very high operating temperatures. In order to capture a maximum amount of energy in usable form requires a large number of nearly reversible steps. Living organisms use a series of specialized protein enzymes which each catalyze small changes in the glucose, often with the assistance of other cofactors which often can be thought of as transporters. Three of the most important are the nucleic acid molecules ATP (adenosine tri-phosphate, which transports energy), NAD (nicotinamide adenine dinucleotide) and FAD (flavin adenine dinucleotide, both which transport energy rich Hydrogen). NAD and FAD each contain a nucleic acid which humans cannot themselves synthesize, so we rely on trace amounts in our food, vitamins called respectively niacin and riboflavin. Contrary to the implication in the above equation, the following steps turning glucose to lactic acid require no oxygen!
But that reaction in a single step would do little more than generate unusable heat energy. The world's best heat engines capture at best roughly a third of the energy into usable work and require very high operating temperatures. In order to capture a maximum amount of energy in usable form requires a large number of nearly reversible steps. Living organisms use a series of specialized protein enzymes which each catalyze small changes in the glucose, often with the assistance of other cofactors which often can be thought of as transporters. Three of the most important are the nucleic acid molecules ATP (adenosine tri-phosphate, which transports energy), NAD (nicotinamide adenine dinucleotide) and FAD (flavin adenine dinucleotide, both which transport energy rich Hydrogen). NAD and FAD each contain a nucleic acid which humans cannot themselves synthesize, so we rely on trace amounts in our food, vitamins called respectively niacin and riboflavin. Contrary to the implication in the above equation, the following steps turning glucose to lactic acid require no oxygen!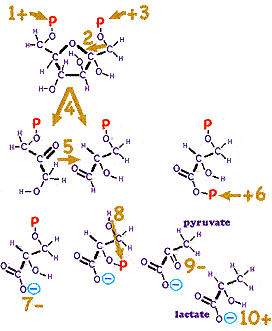
With minor variation, organisms from humans to simple bacteria share the glycolysis process to extract a bit of energy available in glucose. Some bacteria remove from pyruvate the carboxyl group made unstable by the adjacent carbonyl functional group forming ethanol. In higher organisms such as humans, this occurs after step 10 forming the acetate ion. This elaborate process has so far broken the glucose in half, used no Oxygen, and provided only 5% of the energy-transporting ATP that will eventually be generated. The above process was determined in the 1920s and 1930s by joint efforts of Meyerhof, Embden, Parnas, von Euler, Otto Warburg and the Coris, who built on the earlier work of Harden and of Neuberg.
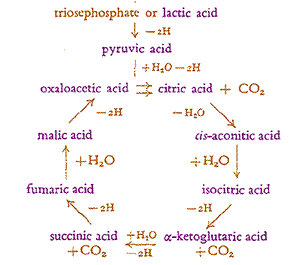
 The Krebs cycle is named after Hans Adolf Krebs (b 1900, d 1981, Nobel photo at right →), former student of Otto Warburg, who proposed the main parts of this cyclic mechanism (his at left ←) in 1937. Krebs was awarded half the 1953 Nobel Prize in Medicine. The first step in the process combines the acetate ion, still attached to coenzyme A to another molecule forming citric acid. So Krebs called this cycle of reactions which represent the main energy source in higher organisms the citric acid cycle.
The Krebs cycle is named after Hans Adolf Krebs (b 1900, d 1981, Nobel photo at right →), former student of Otto Warburg, who proposed the main parts of this cyclic mechanism (his at left ←) in 1937. Krebs was awarded half the 1953 Nobel Prize in Medicine. The first step in the process combines the acetate ion, still attached to coenzyme A to another molecule forming citric acid. So Krebs called this cycle of reactions which represent the main energy source in higher organisms the citric acid cycle.
Early development came from the laboratory of Albert Szent-Györgyi (b1893, d1986) of Szegedin 1935, who discovered that pigeon breast muscle, the powerful flight muscle, is especially suitable for the investigation. He confirmed the rapid oxidation of the four Carbon dicarboxylic acids, succinic, fumaric, malic, and oxaloacetic acids, and arrived at a key conclusion that these substances also had a catalytic effect. The crucial experiments have been repeated with many other animal materials and they suggest that the cycle occurs in all respiring tissues of all animals, from protozoa to the highest mammal.
In the more complete current version of the cycle (below right), the pyruvate has transfered two Hydrogen to NAD resulting in the unstable end carboxyl group next to the carbonyl group braking off to form CO2. The remaining acetate ion, CH3COO-, reacts with a Sulfur atom on Coenzyme A. This joins with a four Carbon remnant on the previous cycle, oxaloacetate (at the 10 o'clock diagram position) forming citrate ion, the tart citric acid abundant in citrus fruit. (The convention presumes Carbon atoms at the unlabeled bond ends, with Hydrogen completing any remaining of Carbon's four bonds.) Here the citrate immediately shifts the hydroxyl group to a more stable position, followed by the rapid release of TWO CO2 made less stable by nearby Oxygen (diagrammed at 1 o'clock and 2 o'clock positions). The remainder of the cycle transfers any of the original glucose's remaining Hydrogen to NAD and restores the oxaloacetate for reuse. The Krebs Cycle turns all of the Carbon in the original glucose to CO2 and exports all the Hydrogen via the cofactors NAD and FAD. Nothing remains of the original food molecule! Another 5% of the energy in glucose is transfered to GTP (like ATP but using a different nucleic acid). Still no Oxygen has been consumed.
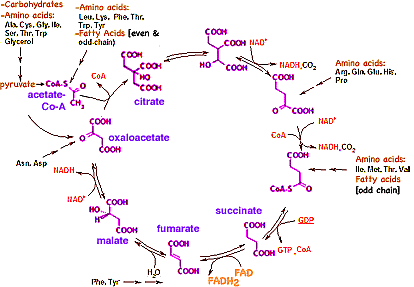 The Krebs Cycle is also used for the metabolism of other food components.
The Krebs Cycle is also used for the metabolism of other food components.
It is remarkable that all foodstuffs are burnt through a common terminal pathway. This makes it possible to eat a wide variety of foods and extract a high percentage of the stored energy, while requiring a minimum number of enzymes to do so.
In his Nobel acceptance speech, Krebs closed with the following remarks:The study of intermediary metabolism shows that the basic metabolic processes, in particular those providing energy, and those leading to the synthesis of cell constituents are ...shared by all forms of life. The existence of common features in different forms of life indicates some relationship between the different organisms, and according to the concept of evolution these relations stem from the circumstance that the higher organisms, in the course of millions of years, have gradually evolved from simpler ones. The concept of evolution postulates that living organisms have common roots, and in turn the existence of common features is powerful support for the concept of evolution. The presence of the same mechanism of energy production in all forms of life suggests two other inferences, firstly, that the mechanism of energy production has arisen very early in the evolutionary process, and secondly, that life, in its present forms, has arisen only once.
The other half of the 1953 Nobel Prize in Physiology or Medicine was awarded to Fritz Albert Lipmann (b1899, d 1986) for his discovery of co-enzyme A which activates a number of the intermediaries in metabolism, making their further reaction more likely. But perhaps more importantly he determined between 1939-1941 that ATP is the universal carrier of chemical energy in the cell. The synthesis of that is outlined in the next section.
While a small amount of ATP is generated in cell cytoplasm in living cells, most ATP is produced in bacteria-like mitochondria which live inside most cells. Probably early bacteria effective in capturing energy infected in a symbiotic relation other single cell organisms which had developed the adaptive advantage of sexual reproduction. The mitochondria, living inside their host cells, reproduce asexually without variation, independent of the sexual reproduction of their host. The mitochondria's DNA sequence is significantly different from the DNA in their host's nucleus. In higher animals, all mitochondria originate from mitochondria in the female parent, with no mitochondria or their DNA transferred from sperm. The mitochondria in all animals, plants, fungi, algae and some bacteria share nearly identical DNA suggesting such a common origin. Mitochondria appear to be the descendants of primitive rickettsia-like-bacteria that developed early an efficient process for converting food energy into ATP. The protein enzymes in the membranes inside the mitochondria are nearly identical to those in plant chloroplasts which transform the energy of sunlight into ATP suggesting both organelles have a common ancestor and a similar history of invading host cells.
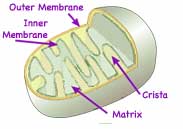 A mitochondrion has both a smooth jelly bean-like outer membrane and a much folded inner membrane. In 1994 Alberts established that the outer membrane contains relatively large protein channels which permit passage of all molecules up to 5000 Daltons. The inner membrane, which is rich in a phospholipid, cardiolipin, common to bacteria membranes but otherwise rare in other cell membranes, is nearly impenetrable, requiring gated proteins for passage of nearly all molecules and ions. The large surface of the folded inner membrane, the cristae, contains embedded proteins with three related functions:
A mitochondrion has both a smooth jelly bean-like outer membrane and a much folded inner membrane. In 1994 Alberts established that the outer membrane contains relatively large protein channels which permit passage of all molecules up to 5000 Daltons. The inner membrane, which is rich in a phospholipid, cardiolipin, common to bacteria membranes but otherwise rare in other cell membranes, is nearly impenetrable, requiring gated proteins for passage of nearly all molecules and ions. The large surface of the folded inner membrane, the cristae, contains embedded proteins with three related functions:
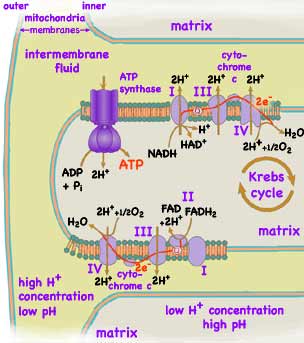 Kreb's citric acid cycle takes place within the mitochondria's fluid inner matrix inside eukaryotes just as it does in the cytoplasm of prokaryotes. The NADH and FADH2 transfer the Hydrogen obtained from the glycolosis and Kreb's cycle to proteins embedded in the cristae. The Hydrogen and NAD+ ions and FAD are released while two associated electrons are transfered along a collection of four protein complexes (numbered I where NADH docks, II where FADH2 docks, III, IV, using a lipid-soluble carrier, Q, and a water-soluble carrier, cytochrome c.) pumping Hydrogen ions into the intermembrane fluid between the inner and outer membranes. The resulting concentration of Hydrogen ions temporarily stores energy. By allowed the concentrated Hydrogen ions to flow back into the inner matrix, another protein complex, ATP synthase, harvests that stored energy. By bonding essentially an inorganic phosphate ion to ADP producing ATP, the energy is transfered into a molecular form suitable for transport and medium duration storage. At the very last step in the metabolism process, the Hydrogen ion and its electron are combined with Oxygen to form water. Note that cells isolate the rather corrosive Oxygen by restricting it to this location and single step. In 1961 Peter D. Mitchell, (b 1920, d 1992, Nobel photo below left) proposed a difference in Hydrogen ion concentration (pH) inside and outside the mitochondrial membrane, and that the stream of Hydrogen ions from high concentration to lower concentration drives the formation of ATP. He showed that the same applies to the chloroplast membrane. He won the 1978 Nobel Prize for Chemistry for proposing and helping to verify this mechanism. Paul D. Boyer (b 1918, Nobel photo below center) and John E. Walker (b 1941, Nobel photo below right) later determined the structure and mechanism of the ATP synthase protein which actually assembles the ATP. They shared half of the 1997 Nobel Prize in Chemistry for their work.
Kreb's citric acid cycle takes place within the mitochondria's fluid inner matrix inside eukaryotes just as it does in the cytoplasm of prokaryotes. The NADH and FADH2 transfer the Hydrogen obtained from the glycolosis and Kreb's cycle to proteins embedded in the cristae. The Hydrogen and NAD+ ions and FAD are released while two associated electrons are transfered along a collection of four protein complexes (numbered I where NADH docks, II where FADH2 docks, III, IV, using a lipid-soluble carrier, Q, and a water-soluble carrier, cytochrome c.) pumping Hydrogen ions into the intermembrane fluid between the inner and outer membranes. The resulting concentration of Hydrogen ions temporarily stores energy. By allowed the concentrated Hydrogen ions to flow back into the inner matrix, another protein complex, ATP synthase, harvests that stored energy. By bonding essentially an inorganic phosphate ion to ADP producing ATP, the energy is transfered into a molecular form suitable for transport and medium duration storage. At the very last step in the metabolism process, the Hydrogen ion and its electron are combined with Oxygen to form water. Note that cells isolate the rather corrosive Oxygen by restricting it to this location and single step. In 1961 Peter D. Mitchell, (b 1920, d 1992, Nobel photo below left) proposed a difference in Hydrogen ion concentration (pH) inside and outside the mitochondrial membrane, and that the stream of Hydrogen ions from high concentration to lower concentration drives the formation of ATP. He showed that the same applies to the chloroplast membrane. He won the 1978 Nobel Prize for Chemistry for proposing and helping to verify this mechanism. Paul D. Boyer (b 1918, Nobel photo below center) and John E. Walker (b 1941, Nobel photo below right) later determined the structure and mechanism of the ATP synthase protein which actually assembles the ATP. They shared half of the 1997 Nobel Prize in Chemistry for their work.
Each NADH molecule generated from a glucose molecule results in 6 H+ ions being pumped across the inner membrane resulting in the formation of 3 ATP molecules. Each FADH2 molecule results in 4 H+ being pumped across the membrane eventually resulting in the production of 2 ATP. As a result, the 10 NADH molecules from glycolysis and the Krebs cycle and the 2 FADH2 molecules form a total of 34 ATP during aerobic respiration. Combining the results of the Krebs Cycle and glycolysis with the 4 ATP produced directly by glycolosis, the 38 ATP contain about 65% of the energy released by the oxidation of glucose to 6 CO2 and 6 H2O. This compares to only 3.5% efficiency for glycolysis alone (as used by primitive organisms) and 35% to 40% for the best thermal power plants burning organic fuel. Note that the multitude of small steps results in living cells capturing far more of the available energy than the best human designed engines.



A fundamental challenge is that food crops grow during the warmer, sunnier Summer and are harvested in the Fall, but are needed for our nourishment year round. Typically food is consumed by numerous competing organisms, many which thereby spoil the food for human consumption. Sauerkraut is an product of cabbage which, by making it unusable by many micro-organisms, preserves most of its nutritious value for human consumption. While common in northern Europe, similar products were also developed in Asia. The cabbage is prepared in such a way that bacteria first are allowed to consume a small portion of the sugar, producing by glycolosis lactic acid which lowers the pH enough to both kill the bacteria and make it an unsuitable living environment for other micro-organisms which might otherwise consume (and spoil) the food. The net reaction is (first generally, then more precisely):
Glucose → Lactic Acid + ATP (Energy)
C6H12O6 + 2 ADP + 2 Pi → C3H6O3 + 2 ATP
But while sugar is neutral, not contributing to the pH, lactic acid is a weak acid so a portion of it ionizes in aqueous solutions, lowering the pH by releasing Hydrogen ions:
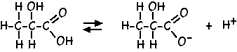
It is relatively easy to make sauerkraut either to eat and/or to study the pH change and/or measure the amount of lactic acid produced.
Communicating technical information such as observations and findings is a skill used by scientists but useful for most others. If you need course credit, use your observations in your journal to construct a formal report.
![]()
to next investigation
to Biochemistry menu
to e-Chemistry menu
to site menu