![]()
![]()
Earlier investigations repeated mentioned the need for energy to power various life processes. Living things do no create energy; energy is conserved and cannot be created or destroyed. Since energy, like most other things, can spontaneously spread out or be shared with its surroundings, the trick is for living organisms to either capture it as is passes by or to harvest energy from a source of concentrated potential energy. Plants capture energy spreading out from the Sun carried by electromagnetic waves we label visible light. The net reaction producing the energy rich carbohydrate glucose with Oxygen as a byproduct is:
6 CO2 + 6 H2O + photons of light (2,870 kJ) → C6H12O6 + 6 O2
Photosynthesis (Greek: photos = a light, syn = together, tithenai = to place) occurs in plants, cyanobacteria and some algae and protists, commonly in specialized organelles called chloroplasts. This processes occurs in two stages: First light is captured and use it to make energy carrying molecules. These are then used to capture carbon dioxide (CO2) and make glucose. The actual chemical mechanisms which are carried out by proteins embedded in membranes are complex.
 While still in England in the 1770s, Joseph Priestley (at left, b1733 in Yorkshire, d1804 in Pennsylvania) performed experiments on various qualities of air showing that plants improve the capacity of air to support combustion. He first fixed the air by burning a candle in a closed vessel until the flame went out. He found that after leaving a sprig of mint in the chamber several days, the air could again suport the candle burning. Although air was still considered one of the four elements, Priestley's work helped overthrow that idea. It was soon realized that air is a combination of substances with one of them, Oxygen, reacted by combustion to form CO2. Plants release the Oxygen from CO2. The Dutch physician Jan Ingenhousz demonstrated that sunlight was necessary for photosynthesis and that only the green plant parts release oxygen. About the same time Jean Senebier, a Swiss botanist and naturalist, discovered that CO2 is required for plant growth and Nicolas-Thodore de Saussure, a Swiss chemist and plant physiologist, showed that water is also required. Not until 1845 did German physician and physicist Julius Robert von Mayer help develop the concept of energy and propose that plants convert light energy into chemical energy.
While still in England in the 1770s, Joseph Priestley (at left, b1733 in Yorkshire, d1804 in Pennsylvania) performed experiments on various qualities of air showing that plants improve the capacity of air to support combustion. He first fixed the air by burning a candle in a closed vessel until the flame went out. He found that after leaving a sprig of mint in the chamber several days, the air could again suport the candle burning. Although air was still considered one of the four elements, Priestley's work helped overthrow that idea. It was soon realized that air is a combination of substances with one of them, Oxygen, reacted by combustion to form CO2. Plants release the Oxygen from CO2. The Dutch physician Jan Ingenhousz demonstrated that sunlight was necessary for photosynthesis and that only the green plant parts release oxygen. About the same time Jean Senebier, a Swiss botanist and naturalist, discovered that CO2 is required for plant growth and Nicolas-Thodore de Saussure, a Swiss chemist and plant physiologist, showed that water is also required. Not until 1845 did German physician and physicist Julius Robert von Mayer help develop the concept of energy and propose that plants convert light energy into chemical energy.
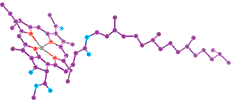
 Molecules of chlorophyll (Greek: chloros = green, phyllon = leaf) absorb predominately red and blue visible light (reflecting green). Hans Fischer (b1881, d1945) was awarded the 1930 Nobel Prize for Chemistry for determining the structure of the molecules of the most fundamental processes of life, the porphyrins in blood and leaf pigments: heme and chlorophyll (the latter at left ←: C, O, N, Mg, with invisible H; plants also have a 2nd form with more O on left edge; several algae forms lack the hydrocarbon tail). The absorption of light by chlorophyll results in the release of an electron which passes along a series of proteins called the electron transport chain which produce the Hydrogen carrier NAD (nicotinamide and adenine, a nucleotide duo) and the energy carrier ATP (adenosine with three energy rich phosphates). The chlorophyll regains an electron from water in a process that releases Oxygen as a byproduct. This happens in pouch shaped membranes inside the chloroplast. Because the probability of capturing a particular photon is low, additional chlorophyll and several other pigments including β-carotene (above right→ C, H), and xanthophyll organized in clusters in the membrane, each absorbing a slightly different band of light colors. These act as receiving antennae and pass the energy to the central chlorphyll associated with the actual photosynthesis process. The central part of a chlorophyll molecule is the porphyrin ring, which consists of several fused rings of Carbon and Nitrogen with a Magnesium ion in the center.
Molecules of chlorophyll (Greek: chloros = green, phyllon = leaf) absorb predominately red and blue visible light (reflecting green). Hans Fischer (b1881, d1945) was awarded the 1930 Nobel Prize for Chemistry for determining the structure of the molecules of the most fundamental processes of life, the porphyrins in blood and leaf pigments: heme and chlorophyll (the latter at left ←: C, O, N, Mg, with invisible H; plants also have a 2nd form with more O on left edge; several algae forms lack the hydrocarbon tail). The absorption of light by chlorophyll results in the release of an electron which passes along a series of proteins called the electron transport chain which produce the Hydrogen carrier NAD (nicotinamide and adenine, a nucleotide duo) and the energy carrier ATP (adenosine with three energy rich phosphates). The chlorophyll regains an electron from water in a process that releases Oxygen as a byproduct. This happens in pouch shaped membranes inside the chloroplast. Because the probability of capturing a particular photon is low, additional chlorophyll and several other pigments including β-carotene (above right→ C, H), and xanthophyll organized in clusters in the membrane, each absorbing a slightly different band of light colors. These act as receiving antennae and pass the energy to the central chlorphyll associated with the actual photosynthesis process. The central part of a chlorophyll molecule is the porphyrin ring, which consists of several fused rings of Carbon and Nitrogen with a Magnesium ion in the center.
In the second stage, CO2 is captured from the atmosphere and converted into carbohydrates in a cyclic process determined by Melvin Calvin (b1911, d1997, at right) at the Lawrence Laboratory in Berkeley. Calvin was awarded the 1961 Nobel Prize in Chemistry for using Carbon-14 as a tracer to determine successive intermediaries as Carbon absorbs atmospheric CO2 and convert it into 3-Carbon sugars which eventually combine into glucose and other organic compounds. Even before 1937 Calvin had become interested in coordination compounds containing metal anions, particularly the Iron coordinated heme and Magnesium coordinated chlorophyll. When Calvin arrived at Berkeley he was encouraged by Gilbert Lewis to try to understand how chlorophyll and its related compounds use light to drive the synthesis of carbohydrates.
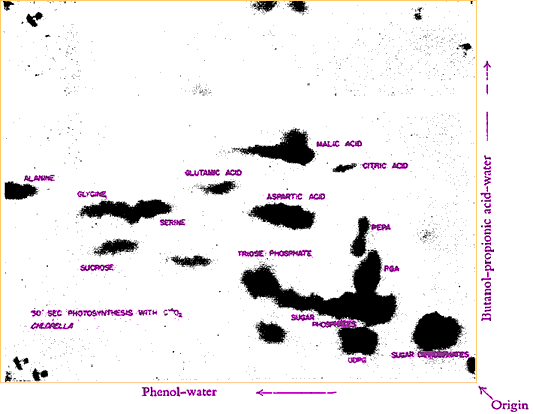 While early chemists presumed that light probably energizes the breakup of CO2 to release the Oxygen and enable the combination of the Carbon with water and subsequently into carbohydrates, there were suspicions that portions of the process might occur in the dark, not directly needing energy from the light. The discovery of the long-lived isotope Carbon-14 by Samuel Ruben and Martin Kamen in 1940 provided an ideal tool for tracing of the route between carbon dioxide and carbohydrate. By exposing plants to light in the absence of CO2 and then moving them into the dark with an atmosphere of 14C*O2, Calvin and his team found that the energized plants could incorporate relatively large amounts of 14C*O2. This refuted the previous assumption and instead confirmed that incorporation of Carbon into organic material is a dark reaction. Working with green algae they next provided 14C*O2 for varying periods of time before killing the cells in alcohol and searching for any synthesized radioactive compounds. They found that the first molecules made were acidic and could be separated by anionic exchange columns. That procedure was expanded to two-dimensional separations on chromatography paper. They confirming suspected products by producing identical chromatography separations starting with known substances. (Chromatograph shows the products formed in 30 seconds. Photograph was made by placing film directly on a chromatograph containing radioactive 14C* compounds. Labels were added later.)
While early chemists presumed that light probably energizes the breakup of CO2 to release the Oxygen and enable the combination of the Carbon with water and subsequently into carbohydrates, there were suspicions that portions of the process might occur in the dark, not directly needing energy from the light. The discovery of the long-lived isotope Carbon-14 by Samuel Ruben and Martin Kamen in 1940 provided an ideal tool for tracing of the route between carbon dioxide and carbohydrate. By exposing plants to light in the absence of CO2 and then moving them into the dark with an atmosphere of 14C*O2, Calvin and his team found that the energized plants could incorporate relatively large amounts of 14C*O2. This refuted the previous assumption and instead confirmed that incorporation of Carbon into organic material is a dark reaction. Working with green algae they next provided 14C*O2 for varying periods of time before killing the cells in alcohol and searching for any synthesized radioactive compounds. They found that the first molecules made were acidic and could be separated by anionic exchange columns. That procedure was expanded to two-dimensional separations on chromatography paper. They confirming suspected products by producing identical chromatography separations starting with known substances. (Chromatograph shows the products formed in 30 seconds. Photograph was made by placing film directly on a chromatograph containing radioactive 14C* compounds. Labels were added later.)
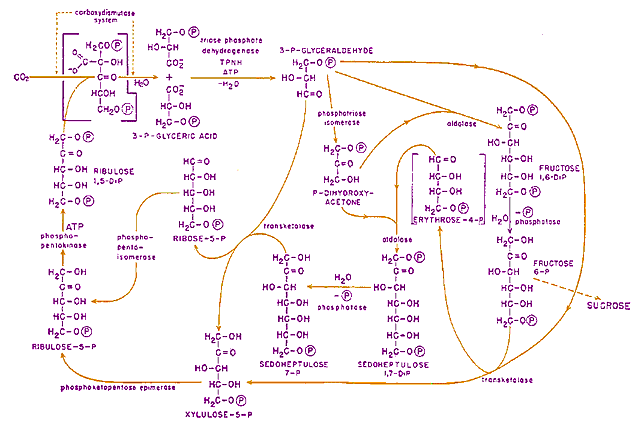 So in the dark, CO2 (entering from the upper left corner in this diagram from Calvin's Nobel talk) attaches to the second carbon (from the top) in a 5 Carbon sugar. This new 6-Carbon sugar immediately separates into two 3-Carbon 3-P-glyceric acid molecules. Then with energy carried by ATP molecules, the carboxyl (-CO2- acid) functional groups are reduced forming 3-P-glyceraldehyde which provides the basis for forming a number of other carbohydrates show at the right and bottom of the diagram. A portion is made into the 5-Carbon ribulose-5-P and then the precursor which can repeat the process.
So in the dark, CO2 (entering from the upper left corner in this diagram from Calvin's Nobel talk) attaches to the second carbon (from the top) in a 5 Carbon sugar. This new 6-Carbon sugar immediately separates into two 3-Carbon 3-P-glyceric acid molecules. Then with energy carried by ATP molecules, the carboxyl (-CO2- acid) functional groups are reduced forming 3-P-glyceraldehyde which provides the basis for forming a number of other carbohydrates show at the right and bottom of the diagram. A portion is made into the 5-Carbon ribulose-5-P and then the precursor which can repeat the process.
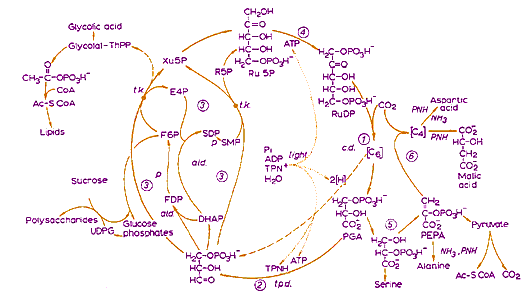 In a broader scheme in Calvin's Nobel talk (shown at left), the 3-Carbon molecule is used to synthesize the organic molecules needed for all plant life. In this diagram the CO2 enters at the 2 o'clock position (numbered 1). Note the sucrose (table sugar), polysaccharides (starch, cellulose, etc.) and lipids produced (on the left) and several amino acids (on the right, used to make protein).
In a broader scheme in Calvin's Nobel talk (shown at left), the 3-Carbon molecule is used to synthesize the organic molecules needed for all plant life. In this diagram the CO2 enters at the 2 o'clock position (numbered 1). Note the sucrose (table sugar), polysaccharides (starch, cellulose, etc.) and lipids produced (on the left) and several amino acids (on the right, used to make protein).
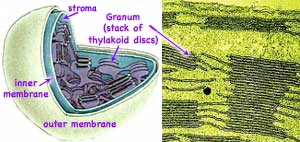 Understanding the light capture has turned out an even larger challenge. Chloroplasts have much in common with today's cyanobacteria including very similar DNA. Perhaps early in the evolution of life, primitive cyanobacteria, having developed a method of capturing sunlight, infected what eventually became plant cells. The symbiotic relationship was mutually advantageous to both with today's chloroplasts being the descendants of the infecting bacteria. The prominent feature in both chloroplasts and cyanobacteria are granum (Latin: grana = stack of coins), stacks of thylakoids (Greek: thulakos = sac) which are composed of double lipid membranes. These membranes are rich in protein complexes containing an abundance of green chlorophyll. The capture of light and the manufacture of hydrocarbons occurs at these thylakoids.
Understanding the light capture has turned out an even larger challenge. Chloroplasts have much in common with today's cyanobacteria including very similar DNA. Perhaps early in the evolution of life, primitive cyanobacteria, having developed a method of capturing sunlight, infected what eventually became plant cells. The symbiotic relationship was mutually advantageous to both with today's chloroplasts being the descendants of the infecting bacteria. The prominent feature in both chloroplasts and cyanobacteria are granum (Latin: grana = stack of coins), stacks of thylakoids (Greek: thulakos = sac) which are composed of double lipid membranes. These membranes are rich in protein complexes containing an abundance of green chlorophyll. The capture of light and the manufacture of hydrocarbons occurs at these thylakoids.
In 1954 Daniel Arnon and coworkers discovered that plants, and A. Frenkel discovered that photosynthetic bacteria, use light energy to produce ATP. About the same time L.N.M. Duysens showed that the primary photochemical reaction of photosynthesis is an oxidation/reduction reaction that occurs in a protein complex. Over the next few years the work of several groups, including those of Robert Emerson, Bessel Kok, L.N.M. Duysens, Robert Hill and Horst Witt, found that plants, algae and cyanobacteria require two protein complexes, called photosystem I and photosystem II, operating in series. In 1961 Peter Mitchell (b1920, d1992) suggested that cells store energy by creating an electric potential (measured as Voltage) across membranes. He suggested they do this by pumping H+ (= protons) across membranes making a concentration gradient with associated osmotic pressure. Mitchell's proposal clarified how protein complexes embedded in membranes store and ultilize energy in mitochondria and chloroplasts and other systems. Mitchell was awarded the Nobel Prize in Chemistry in 1978 for his theory of chemiosmotic energy transduction. But efforts to determine the structure of these membrane proteins and the mechanisms they use had little success for several decades.
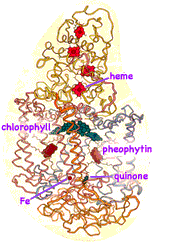 Johann Deisenhofer (b1943, Howard Hughes Medical Institute, Dallas), Robert Huber (b1937, Max-Planck-Institut für Biochemie, Martinsried, Germany) and Hartmut Michel (b1948, Max-Planck-Institut für Biophysik, Frankfurt/Main) were awarded the 1988 Nobel Prize in Chemistry for determining the structure of the photosynthetic reaction complex in the purple bacterium Rhodospeudomonas viridis. By substituting a detergent similar to the double lipid membrane, they were able to extract, dissolve and crystalize the protein complex making it solid enough to study its structure by X-ray diffraction. The complex (shown at left) is composed of four proteins, two each have five-membrane crossing segments, while a third is anchored to the inner membrane surface (bottom of these diagrams) by a single helical segment, and the fourth binds to the outer surface of the membrane. Four chlorophyll, two pheophytin (chlorophyll-like molecules but lacking Magnesium), and a quinone occupy the reaction center in the middle of the complex.
Johann Deisenhofer (b1943, Howard Hughes Medical Institute, Dallas), Robert Huber (b1937, Max-Planck-Institut für Biochemie, Martinsried, Germany) and Hartmut Michel (b1948, Max-Planck-Institut für Biophysik, Frankfurt/Main) were awarded the 1988 Nobel Prize in Chemistry for determining the structure of the photosynthetic reaction complex in the purple bacterium Rhodospeudomonas viridis. By substituting a detergent similar to the double lipid membrane, they were able to extract, dissolve and crystalize the protein complex making it solid enough to study its structure by X-ray diffraction. The complex (shown at left) is composed of four proteins, two each have five-membrane crossing segments, while a third is anchored to the inner membrane surface (bottom of these diagrams) by a single helical segment, and the fourth binds to the outer surface of the membrane. Four chlorophyll, two pheophytin (chlorophyll-like molecules but lacking Magnesium), and a quinone occupy the reaction center in the middle of the complex.
The antenna system consists of hundreds of pigment molecules which are anchored to the proteins. The absorption of a photon of light by an antenna molecule occurs in 10-15 femtoseconds and causes a transition from an electron's ground state to an excited state. The excited electron decays back to ground energy within a few hundred picoseconds by transferring its energy via resonance vibration along the antenna system to the reaction center. There the central chlorophyll emits an electron to the pheophytin. Less than a billionth of a second later an electron is transferred to the quinone inside the complex. Within a millionth of a second the chlorophyll is reduced (gains a replacement electron) and is again ready to adsorb light energy. Meanwhile, the ejected electron is kept from returning and instead transferred out of the complex.
Much of our current understanding of the physical principles that guide electron transfer is based on the pioneering work of Rudolph A. Marcus, (b1923 in Montreal) who received the Nobel Prize in Chemistry in 1992 for his contributions to the theory of electron transfer reaction in chemical systems.
The chlorophylls in Photosystem II and Photosystem I have different absorption peaks and so are different shades of green. They are called P680 and P700 respectively after the wavelengths (in nm) of their first (red) absorption peak. The light photosystem II feeds electrons to photosystem I by intermediate carriers.
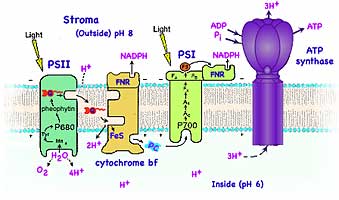 Photosystem II uses the captured light energy to drive the oxidation of water and the reduction of quinone. The photosystem II complex involves more than 15 proteins and at least 9 different redox components (chlorophyll, pheophytin, quinone, tyrosine, Mn, Fe, cytochrome b, carotenoid and histidine). Five of these redox components are involved in transferring electrons from H2O to quinone: a water oxidizing Manganese cluster, the amino acid tyrosine, the P680 reaction center chlorophyll, pheophytin, and quinone.
Photosystem II uses the captured light energy to drive the oxidation of water and the reduction of quinone. The photosystem II complex involves more than 15 proteins and at least 9 different redox components (chlorophyll, pheophytin, quinone, tyrosine, Mn, Fe, cytochrome b, carotenoid and histidine). Five of these redox components are involved in transferring electrons from H2O to quinone: a water oxidizing Manganese cluster, the amino acid tyrosine, the P680 reaction center chlorophyll, pheophytin, and quinone.
Photochemistry in photosystem II starts with the transfer of an electron from P680 to pheophytin. This charge separation takes about a few picoseconds. The electron on a quinone inside the reaction center is then transferred to a second quinone molecule that is loosely docked at the side of the complex near the outside of the thylakoid. H+ from outside of the membrane bond to the quinone as it gains the electron. This reduced quinone undocks from the reaction center and diffuses through the non-polar hydrocarbon chains of the lipids in the membrane while a replacement oxidized quinone molecule finds its way to the docking site preparing the process for repetition.
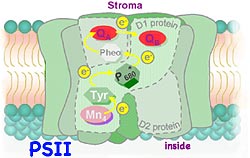 Meanwhile at the bottom on the complex, electrons are removed from water to replace the electron ejected by the P680 chlorophyll, releasing O2 and H+ ions into the interior of the thylakoid. Plants, algae and certain bacteria all have photosystem II which share this structure and function. (Four other phyla, Purple Bacteria, Green Sulfur Bacteria, Green Gliding Bacteria, and Gram Positive Bacteria, use light energy to extract electrons from molecules other than water. These organisms are ancient and are believed to have evolved before the Oxygen generating organisms.) Photosystem II is the only known protein complex that can oxidize water, releasing O2 into the atmosphere. The mechanism for water oxidation is not fully understood. Powerful oxidants removing electrons from water but how the electrons are transferred from water to reduce the P680 remains unclear. In bright light a single reaction center may capture light energy at a rate as great as 600 eV/s (equivalent to 60,000 kW per mole of PS II). Discovering how to replicate such energy transfer efficiency would significantly help solve the world's energy problems.
Meanwhile at the bottom on the complex, electrons are removed from water to replace the electron ejected by the P680 chlorophyll, releasing O2 and H+ ions into the interior of the thylakoid. Plants, algae and certain bacteria all have photosystem II which share this structure and function. (Four other phyla, Purple Bacteria, Green Sulfur Bacteria, Green Gliding Bacteria, and Gram Positive Bacteria, use light energy to extract electrons from molecules other than water. These organisms are ancient and are believed to have evolved before the Oxygen generating organisms.) Photosystem II is the only known protein complex that can oxidize water, releasing O2 into the atmosphere. The mechanism for water oxidation is not fully understood. Powerful oxidants removing electrons from water but how the electrons are transferred from water to reduce the P680 remains unclear. In bright light a single reaction center may capture light energy at a rate as great as 600 eV/s (equivalent to 60,000 kW per mole of PS II). Discovering how to replicate such energy transfer efficiency would significantly help solve the world's energy problems.
As the electron ejected by the captured light energy is transfered between proteins embedded in the lipid membrane, some of its energy is used to concentrate H+ ions inside the thylakoid. The resulting concentration gradient (with more H+ inside the thylakoid and less outside) creates an electric field across the membrane containing the photosystem complexes. Thus the electron transfer converts some of the light's energy captured by the oxidation-reduction reaction into potential energy stored in the electrical potential of the concentration of Hydrogen ions. While these protons might be thought of as charge carriers much tinier than any atom, they actually are enveloped by shared electron clouds while bonded to the quinone as well as when they are hydrated in the aqueous solutions inside or outside the thylakoid.
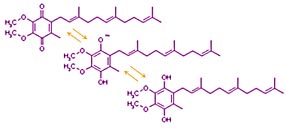 Photosysthesis uses metal ion coordination complexes (metal ions which lack valence electrons sharing electron pairs contributed by nearby atoms which have otherwise unshared electron pairs) and aromatic groups (organic molecules with alternating double and single bonds which can transfer charge by trading locations) to transport electrons. The metal ion complexes and most of the aromatic groups are bound within the protein complexes. But electron carriers such as quinone are not bound to proteins. Quinone's aromatic (alternating double bonds around the ring) allow a negative charge to be shared across the molecule allowing the oxidized form (shown on top) to be easily reduced (to bottom structure). Other quinones have hydrocarbon tails of different lengths. (That shown here has three isoprene units. Others such as Coenzyme Q in mitochondria have 10. Plastoquinone used in photosynthesis has 9.)
Photosysthesis uses metal ion coordination complexes (metal ions which lack valence electrons sharing electron pairs contributed by nearby atoms which have otherwise unshared electron pairs) and aromatic groups (organic molecules with alternating double and single bonds which can transfer charge by trading locations) to transport electrons. The metal ion complexes and most of the aromatic groups are bound within the protein complexes. But electron carriers such as quinone are not bound to proteins. Quinone's aromatic (alternating double bonds around the ring) allow a negative charge to be shared across the molecule allowing the oxidized form (shown on top) to be easily reduced (to bottom structure). Other quinones have hydrocarbon tails of different lengths. (That shown here has three isoprene units. Others such as Coenzyme Q in mitochondria have 10. Plastoquinone used in photosynthesis has 9.)
Electrons are transferred between the large protein complexes by quinone. These small molecules carry electrons and Hydrogen atoms over the relatively long distances between protein complexes. Because quinone is hydrophobic it remains inside the membrane, dissolved in the non-polar hyrdrocarbon chains of the lipids. Quinone diffuses through the membrane until attracted to a site on the photosystem II complex. These small molecules dock at specialized functional groups on the protein surfaces known as binding sites where they are reduced or oxidized and pick up or release Hydrogen ions. The photosystem II reaction center reduces quinone by adding two electrons and two protons. The reduced quinone molecule undocks from photosystem II and diffuses randomly in the photosynthetic membrane until docking at a binding site on the cytochrome b complex.
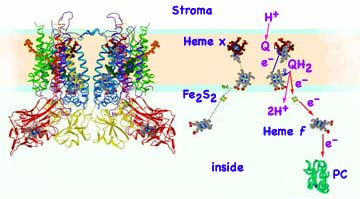 Biochemical analyses and mass spectroscopy of the proton-pumping cytochrome b6f complex found it to be a dimer (each monomer of mass 108,541 Dalton containing 8 subunits in the cyanobacterium M. laminosus and 9 subunits in plant chloroplasts. The extra subunit is ferredoxin-NAD-reductase: FNR). The b6f complex (structure near right) is in many respects analogous in function and structure to the cytochrome bc1 complex of mitochondria. The cytochrome bf protein complex, bound in the lipid membrane with 13 trans-membrane helices per monomer, contains four electron carriers, three cytochromes and an Rieske high potential Iron Sulfur protein (Fe2S2). Three groups, (chlorophyll, β-carotene, and a unique protein-bound heme x with no amino acid side chains) are not found in mitochondria cytochromes. The cytochrome bf complex removes the electrons from quinone then releases of the Hydrogen ions into the aqueous fluid inside the thylakoid. The electrons are eventually transferred to the photosystem I reaction center. The electron and Hydrogen ion pathways (far right, derived from Cramer's) starts with the quinone, Q, picking up the outside Hydrogen ion at PSII and docking while carrying the two Hydrogen, QH2. The central cavity in the b6f dimer is the quinone's binding cavity. The quinone is in reduced form on the open top side of the complex and oxidized in the niche on the bottom of the cavity. To reach this bottom niche, the quinone must pass through a small portal in the complex.
Biochemical analyses and mass spectroscopy of the proton-pumping cytochrome b6f complex found it to be a dimer (each monomer of mass 108,541 Dalton containing 8 subunits in the cyanobacterium M. laminosus and 9 subunits in plant chloroplasts. The extra subunit is ferredoxin-NAD-reductase: FNR). The b6f complex (structure near right) is in many respects analogous in function and structure to the cytochrome bc1 complex of mitochondria. The cytochrome bf protein complex, bound in the lipid membrane with 13 trans-membrane helices per monomer, contains four electron carriers, three cytochromes and an Rieske high potential Iron Sulfur protein (Fe2S2). Three groups, (chlorophyll, β-carotene, and a unique protein-bound heme x with no amino acid side chains) are not found in mitochondria cytochromes. The cytochrome bf complex removes the electrons from quinone then releases of the Hydrogen ions into the aqueous fluid inside the thylakoid. The electrons are eventually transferred to the photosystem I reaction center. The electron and Hydrogen ion pathways (far right, derived from Cramer's) starts with the quinone, Q, picking up the outside Hydrogen ion at PSII and docking while carrying the two Hydrogen, QH2. The central cavity in the b6f dimer is the quinone's binding cavity. The quinone is in reduced form on the open top side of the complex and oxidized in the niche on the bottom of the cavity. To reach this bottom niche, the quinone must pass through a small portal in the complex.
Consuming some of the energy carried by the electrons, the Hydrogen ions released inside the thylakoid are concentrated compared with outside the thylakoid. The resulting pH gradient is used by another membrane protein, ATP synthase (ATPase for short), to add phosphate to ADP forming the energy rich ATP which provides energy as needed for other cell processes.
 The electrons originally released by the capture of light in photosystem II are carried from the cytochrome to the photosystem I protein complex by a small water soluble protein, plastocyanin, containing the Cu+2 ion. While it's structure varies somewhat from one phylum to another, that of the French Bean is shown at left.
The electrons originally released by the capture of light in photosystem II are carried from the cytochrome to the photosystem I protein complex by a small water soluble protein, plastocyanin, containing the Cu+2 ion. While it's structure varies somewhat from one phylum to another, that of the French Bean is shown at left.
Photosystem I is capable of using the absorption of a second photon of light to boost the energy of the electrons from PSII. Originally, a photon was absorbed by the chlorophyll core of photosystem II, exciting two electrons which are transferred to via quinone to the cytochrome. The photosystem I complex removes the electrons (oxidation) from plastocyanin transferring the electrons (reduction) to ferredoxin, a small protein containing FeS. The reaction center is served by an antenna system that consists of about two hundred chlorophyll molecules with a P700 chlorophyll at the reaction center. Primary charge separation occurs between P700 and a chlorophyll monomer. The energy absorbed by the second photon boosts their energy level to a higher level. The highly excited electrons are transferred to ferredoxin, and then to an enzyme called ferredoxin-NAD-reductase, for short FNR, which uses them to drive the reaction:
NADP+ + H+ + 2e- → NADPH
This consumes one of the H+ ions produced by the splitting of water and provides a Hydrogen being carried by nicotinamide adenine dinucleotide (NAD) with sufficient energy to be useful for synthesis of more complex organic molecules in the Calvin cycle. For this electron transfer chain starting with photosystem II to occur, both active centers must absorb light close to simultaneously.
In dimmer light when a photon is absorbed by photosystem I and not by photosystem II, an electron can originate in a pigment complex of photosystem I, pass to ferredoxin, then to a complex of two cytochromes (similar to those found in mitochondria), and then to plastocyanin before returning to chlorophyll. This transport chain pumps Hydrogen ions across the membrane contributing to the pH gradient later used by ATP synthase to make ATP. This pathway produces neither O2 nor NADPH. In bacterial photosynthesis, a single photosystem I is used.
The conversion of energy stored in the concentrated H+ inside the thylakoids into energy stored in molecules of ATP is accomplished by the protein complex known as ATP synthase. This enzyme catalyzes inorganic phosphate, Pi, adding to the two phosphate groups already present in ADP:
ADP + Pi + H+in → ATP + H2O + H+out
The reaction requires 32 kJ/mol of energy and is driven by the osmotic flow of H+ through the ATP synthase protein. The ATP Synthase complex is composed of two distinct proteins. The complex spans the lipid membrane and provides a channel through the membrane for Hydrogen ions. The top portion contains the catalytic sites for ATP synthesis. Three H+ pass through the complex, powering the synthesis of each molecule of ATP, but are not directly involved in adding phosphate to ADP.
The ATP and NADPH made in the light capturing part of photosynthesis is then used to synthesize carbohydrates using the process first understood by Calvin. The NADPH and ATP formed by the light reactions provide the energy for these dark reactions of photosynthesis. The proteins required for the reduction of atmospheric CO2 to carbohydrate are located in the stroma, the aqueous phase outside the thylakoid's photosynthetic membrane. The first step is catalyzed by the protein abbreviated Rubisco (D-ribulose 1,5-bisphosphate carboxylase/oxygenase), which attaches CO2 to a five-Carbon compound. The reaction produces two molecules of a three-carbons each. Subsequent biochemical reactions involve enzymes which reduce Carbon by Hydrogen transfer and rearrange the Carbon compounds to synthesize carbohydrates.
Chromatography (Greek: chroma = colour; graphos = to write) was used by Melvin Calvin to separate the products of dark photosynthesis. The first chromatography technique was invented by the Russian botanist Mikhail Semyonovich Tsvet in 1901 to separate chlorophyll. He used a column containing calcium carbonate to separate plant pigments. The technique which uses the competition between the attraction of a substance for a surface verses that of a solvent is useful when harsher separation techniques might destroy or change the substances being separated. It can be used for 1-dimensional separations by using columns of material or aligned filaments such as in paper, for 2-dimensional separations using uniform solids coated on a flat backing plate, or presumably 3-dimensional separations. A significant amount of the experiment involves finding both suitable separation material and the solvent.
For a first experiment you might try paper chromatography to separate either colored inks or pigments used to color food. Some may be a single colored substance while others may be a mixture of pigments. If you are already familiar with paper chromatography, you might try more challenging chromatographs.
Column chromatography involves uniformly packing a powder inside a long cylinder. A wide variety of powders could be used from powdered sugar to commercially available ion exchange resins. A larger amount of the pigments is applied to the top of the column then one or more solvents are relatively continuously applied. The pigments are dragged through the powder at different rates and can be collected when each emerges at the bottom of the column after different delay times.
Two-dimensional chromatography involves selecting an inert backing material such as a sheet of plastic or glass. A thin layer of coating material such as a gel or painted powder can be spread on the backing. Commercially prepared chromatography sheets are available, but it is possible to experiment and construct your own. A spot is placed near one corner. One edge of the sheet adjacent to the spot is lowered into a container immersing only the edge in a solvent. After the solvent climbs the sheet nearly reaching the top edge, the sheet is removed and allowed to dry. Later the sheet is rotated 90° so that the other edge adjacent to the original spot location is immersed in a different solvent.
Consider what would be required to do 3-dimensional chromatography. Perhaps you could conduct tests using pigments injected into a block of gelatin? Are there other, better ways? What would be the advantages and disadvantages of 3-dimensional chromatography?
A critical part of any experiment involves conceiving the idea for the experiment and planning what should be done. It should be considered normal for this stage to often require significant time and require numerous revisions and adjustments. (Perhaps these processes are largely ignored by traditional science instruction?) It is commonly acknowledged that finding one or more abundant, safe, inexpensive sources of retrievable energy is one of the major problems facing humans. Apparently the largest source available is the energy carried by light from the Sun. But that has the difficulty that it is spread broadly, varies in intensity with time and season, and difficult to collect. Plants have a light capturing mechanism that is moderately efficient, but still not completely understood. Presuming that the remaining aspects will be learned, the challenge might be to figure a way to optimize aspects of photosynthesis to meet human needs. Taking understanding learned by the processes of science and applying that to practical uses is defined as engineering. Although the goals are different, many of the experimental processes used by science are shared with engineering.
Communicating technical information such as observations and findings is a skill used by scientists but useful for most others. If you need course credit, use your observations in your journal to construct a formal report
![]()
to next investigation
to Biochemistry menu
to e-Chemistry menu
to site menu