![]()
On first impression, the climate might not seem like chemistry. But the absorption of energy from the Sun and the emission of radiation back into space is determined by the chemical substances in the atmosphere and on the Earth's surface. Typical substances are transparent to some wavelengths of sunlight while absorbing at other wavelengths. And the surfaces of materials are often reflective under some conditions but not others. Finally, substances often release energy at a different wavelength than they absorb. This might all be of little concern to humans except that human activities have been unintentionally changing the balance of these processes in ways that may be undesirable.
Archaeologists studying ancient civilizations have noticed that human civilizations did not always get progressively better but sometimes declined and occasionally totally collapsed. They have suggested than the declines of a number of human civilizations were at least partially due to failing to correctly understand and adjust to changes in their environment. If that is true, then it may be imperative that we try to understand what changes we are making in our environment and make any adjustments that are appropriate.
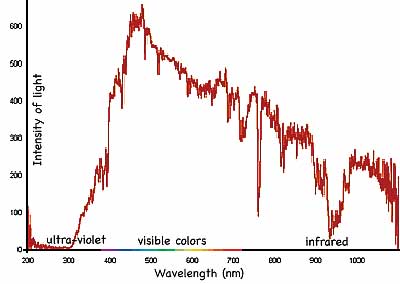 There is evidence that the Sun has emitted a steady rate of energy (at 1.99 calories/cm2/minute = 1.73 x 1017 Watts at a distance of one astronomical unit) for many centuries and will continue doing so (except for a 3% increase every 11 years related to the sunspot cycle). That energy is spread over various wavelengths (or colors) of light in a nearly bell shaped curved (more precisely, a Maxwell-Boltzmann distribution tailing off to right) centering on visible light. The air surrounding the earth is transparent to the visible wavelengths allowing much of the light into the earth. Ozone, O3, and other molecules in the upper atmosphere block nearly all the short wavelength ultraviolet light which otherwise would harm life. Other molecules absorb light at particular colors making the intensity of light reaching the earth pocked by dips (as shown at right). About 25% of the incident solar radiation is absorbed by the atmosphere and turned to heat.
There is evidence that the Sun has emitted a steady rate of energy (at 1.99 calories/cm2/minute = 1.73 x 1017 Watts at a distance of one astronomical unit) for many centuries and will continue doing so (except for a 3% increase every 11 years related to the sunspot cycle). That energy is spread over various wavelengths (or colors) of light in a nearly bell shaped curved (more precisely, a Maxwell-Boltzmann distribution tailing off to right) centering on visible light. The air surrounding the earth is transparent to the visible wavelengths allowing much of the light into the earth. Ozone, O3, and other molecules in the upper atmosphere block nearly all the short wavelength ultraviolet light which otherwise would harm life. Other molecules absorb light at particular colors making the intensity of light reaching the earth pocked by dips (as shown at right). About 25% of the incident solar radiation is absorbed by the atmosphere and turned to heat.
 In addition the water droplets in clouds refract visible light scattering much it of back towards space. Another 25% of the incident solar radiation is reflected back into space. Light colored soil and ice fields reflect about 5% of the incident radiation with the remaining 45% absorbed by the oceans, land, and plants. Only 0.02% is actually captured by photosynthesis of plants and utilized by life. Deforestation, creation of smoke, and other human activities can influence how much of the incident solar radiation is immediately reflected back into space. All the absorbed energy eventually warms various parts of the earth.
In addition the water droplets in clouds refract visible light scattering much it of back towards space. Another 25% of the incident solar radiation is reflected back into space. Light colored soil and ice fields reflect about 5% of the incident radiation with the remaining 45% absorbed by the oceans, land, and plants. Only 0.02% is actually captured by photosynthesis of plants and utilized by life. Deforestation, creation of smoke, and other human activities can influence how much of the incident solar radiation is immediately reflected back into space. All the absorbed energy eventually warms various parts of the earth.
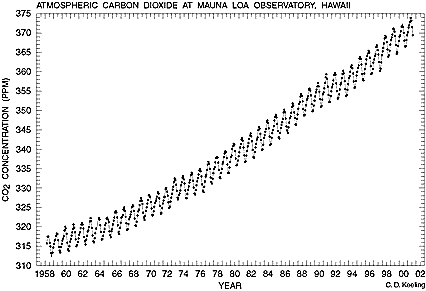 Each warm object eventually releases the energy, emitting infrared light as governed by the object's temperature. But unlike most of the directly reflected light, some of the infrared light can be recaptured by the atmosphere. This effect was first proposed nearly two Centuries ago by Fourier. Greenhouse gases such as CO2 operate by the same mechanism as a greenhouse. Such a building lets visible light in through the window glass which is clear to visible colors but opaque to infrared radiation. Thus the energy is captured inside, warming the building. Likewise the greenhouse gases are transparent to visible light but absorb infrared radiation, warming the earth. In 1896 Svante Arrhenius published a paper On the Influence of Carbonic Acid in the Air upon the Temperature of the Ground presenting calculations on the effect of atmospheric CO2 concentrations on temperature as a function of latitude. He proposed a decline on CO2 concentration could cause an ice age. But this caused little concern for six decades. Charles David Keeling (1928-2005, photo above) was recruited by Scripps Institute of Oceanography in 1956 to help monitor CO2. In 1958 he started measured CO2 on top of Mauna Loa on the island of Hawaii, in the middle of the Pacific Ocean to establish a baseline of CO2 in the earth's atmosphere. He discovered a seasonal variation in concentration (correlated with photosynthesis variations). But his persistence in continuing measurements (for over 40 years) led after a couple years to the far more important discovery that there is a steady annual increase in the earth's CO2 concentration. There is now little doubt that the burning of fossil fuels to power the industrial age is changing the earth's temperature. Keeling's pioneering work fundamentally changed the way we view our role on planet Earth.
Each warm object eventually releases the energy, emitting infrared light as governed by the object's temperature. But unlike most of the directly reflected light, some of the infrared light can be recaptured by the atmosphere. This effect was first proposed nearly two Centuries ago by Fourier. Greenhouse gases such as CO2 operate by the same mechanism as a greenhouse. Such a building lets visible light in through the window glass which is clear to visible colors but opaque to infrared radiation. Thus the energy is captured inside, warming the building. Likewise the greenhouse gases are transparent to visible light but absorb infrared radiation, warming the earth. In 1896 Svante Arrhenius published a paper On the Influence of Carbonic Acid in the Air upon the Temperature of the Ground presenting calculations on the effect of atmospheric CO2 concentrations on temperature as a function of latitude. He proposed a decline on CO2 concentration could cause an ice age. But this caused little concern for six decades. Charles David Keeling (1928-2005, photo above) was recruited by Scripps Institute of Oceanography in 1956 to help monitor CO2. In 1958 he started measured CO2 on top of Mauna Loa on the island of Hawaii, in the middle of the Pacific Ocean to establish a baseline of CO2 in the earth's atmosphere. He discovered a seasonal variation in concentration (correlated with photosynthesis variations). But his persistence in continuing measurements (for over 40 years) led after a couple years to the far more important discovery that there is a steady annual increase in the earth's CO2 concentration. There is now little doubt that the burning of fossil fuels to power the industrial age is changing the earth's temperature. Keeling's pioneering work fundamentally changed the way we view our role on planet Earth.
In 1973 at the Mitre Corporation I received a call from the National Science Foundation asking me to conduct a study under the recently authorized Research Aimed at National Needs program. The study was to develop a research program to foster the use of solar energy. My report, Energy Use and Climate, NSF-RA-N-75-052, was published in April 1975. This was the first U.S. government report stating that the use of solar energy instead of stored energy sources (both fossil and nuclear) would avoid the problem of ... global temperature increase. ... My conclusion was that the use of fossil fuels would have to be sharply curtailed by 2025 and eliminated by 2100 to avoid temperature increases of 2 to 3°C globally and 10°C at the poles. The world is rapidly moving towards those numbers. ... Despite the billions of dollars invested, we have essentially lost 30 years in implementation of significant amounts of solar energy production. ... the only way to make solar energy cost-effective is to legislation a carbon tax or other method to sharply curtail the use of fossil fuels.While this experiment was being drafted in 2005, the U.S. Congress and President considered, passed and signed legislation REDUCING taxes to ENCOURAGE the burning of fossil fuels! And by year end, 2005 had been recorded as the year with
the highest global surface temperature in more than a century of instrumental data..., a fact which the U.S. Bush administration pressured NASA to remove from its website.
There has been much evidence gathered suggesting that the Earth's temperature continues to warm as the levels of carbon dioxide increase. But controversy remains as to how much of the temperature change is due to the CO2 concentration increase and how much is due to other causes. Water vapor, H2O, is responsible for roughly 95 per cent of the total greenhouse effect due to all atmospheric gases. The proportion due to water varies with season, weather, temperature, and other local differences. But many scientists believe that proportion is only temperature related when averaged over the entire Earth. The other greenhouse gases, carbon dioxide, methane (CH4), nitrogen dioxide (NO2), and various others including various halogenated organic molecules, contributed the remaining 5% of the greenhouse effect, with CO2 being the greatest contributor at 3.6%. Human-related methane, nitrogen dioxide and ChloroFluoroCarbons (CFCs, predominately escaping after use as the working fluid in refrigeration devices) contribute 0.066%, 0.047% and 0.046% respectively. Thus with the other factors having tiny effects or relatively constant effects, the increase in carbon dioxide concentration is thought to govern global temperature rise with the temperature effect on water vapor magnifying the warming.
There is also controversy as to whether CO2 emissions should be immediately curtailed. Some people propose that funds should be spent to maximize benefits, to current funding for research with anticipation that more economical solutions will be found.
In pure water near room temperature, the concentration of H+1 is about 1 x 10-7M so that the pH of the water is about 7. Consider vinegar, an acid with concentration of H+1 much GREATER, perhaps 10-2M, the pH is about 2. In household ammonia, a strong base with concentration of H+1 much LOWER, nearly 10-14M, the pH is nearly 14. So small differences in pH numbers represent much larger differences in H+1 concentration. Because of the negative sign in the definition of pH, a DECREASE in pH by one represents a TEN-fold INCREASE. And a LOWER pH by 3 represents 1000 ( = 103) times MORE H+1 concentration.
Because of the substances dissolved in the oceans, the pH of surface sea-water is about 8.2 pH. And due to the increasing CO2 in the atmosphere, more CO2 has dissolved in the oceans lowering over two centuries the pH from 8.3, a 30% increase in H+1 concentration. That increase may be enough to modify the balance of life in the ocean, for example greatly reducing the viability of the great coral reefs. Continued use of fossil fuels may decrease pH 0.5 by the end of the century.
To develop a feel for the effect of CO2 on H+1 concentration in water, we need a method of detecting pH differences. At higher concentrations our human tongues detect H+1 as a tangy sour taste. But at the concentration of tap water or sea-water, our senses need assistance. Fortunately there are chemicals called indicators which change color when immersed in water of different pH. While it is possible to purchase pH indicators, it is reasonably easy to extract natural indicators from fruits and colored vegetables.
In general such an experiment as this involves three stages:Communicating technical information such as observations and findings is a skill used by scientists but useful for most others. If you need course credit, use your observations in your journal to construct a formal report.
![]()
to next investigation
to Environmental Chemistry menu
to ie-Chemistry menu
to site menu