![]()
![]()
This experiment investigates one of the ways protection can be provided from the effects of high energies released by radioactive events.
Humans have no senses which can directly detect radioactive events. Except for rare situations, we normally can't see it (the Cherenkov effect is an exception), smell it, taste it, hear it, or feel it (unless enormous intensity generates noticeable heat). Antoine Henri Becquerel found that radioactivity can change photographic film (X-rays have been photographed by physicians for over a century). Pierre and Marie Curie developed a way of detecting radiation by the rate electric charge leaked off an electrometer. Hans Geiger developed a way to use ionization of a gas by radioactivity to produce electrical pulses. The pulse from his Geiger counters can be portrayed visually on a screen or more often presented as a click on a speaker.
For over 35 years, students in the author's classes have safely performed the following experiment with small amounts of radioactive substances imbedded in plastic disks. The small amount of energy released in a class period is generally less harmful than the slight increase in cosmic ray exposure received when not protected by a roof while outside in Physical Education class! Therefore the students needed no special protective clothing, gloves, or goggles.
Because you probably don't have a Geiger counter, a Geiger counter's electrical signal has been stored in a sound file. So rather than being immediately played through a speaker at the time of the experiment, the stored signal can be played through your computer's speakers. The Geiger counter does not capture the small amount of radiation energy, but instead uses power drawn from an electrical power supply. Likewise none of the radiation energy is contained in the sound file, but rather the energy released from your speakers comes for the same power source that is running your computer.
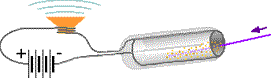
The Geiger counter uses the radiation as a trigger for the electrical signal. Radiation enters the Geiger-Muller tube either through a very thin (mica) window at one end or along one side. Molecules of a gas inside the tube are torn apart forming electrically charged ions either by γ (gamma) radiation electromagnetically colliding with the molecules or α (alpha) and β (beta) radiation doing so with strong magnetic fields. Unlike the originally uncharged molecules, these ions carry electric charge across a gap between the metal cylinder and a central metal wire. A high voltage power source pumps replacement charge to the metal electrodes. If the wires to the electrodes pass around a speaker magnet, a click occurs each time the radiation enters the tube, ionizes the gas, and generates a surge of electric current through the wires. A chemical reaction rapidly regenerates the original molecules so the tube will be ready to detect the next entering radiation.
In this experiment we shall investigate the intensity of radiation at various distances from a radioactive source.
| Measurement # | Radiation Source | sound file | visual plot |
| 1 | background | aiff file, mp3 file | graph |
| 2 | background | aiff file, mp3 file | graph |
| 3 | background | aiff file, mp3 file | graph |
| 4 | background | aiff file, mp3 file | graph |
| 5 | background | aiff file, mp3 file | graph |
| Radiation Source | Distance to Detector | sound file | visual plot |
| Co-60 | 30 cm | aiff file, mp3 file | graph |
| Co-60 | 25 cm | aiff file, mp3 file | graph |
| Co-60 | 20 cm | aiff file, mp3 file | graph |
| Co-60 | 18 cm | aiff file, mp3 file | graph |
| Co-60 | 16 cm | aiff file, mp3 file | graph |
| Co-60 | 14 cm | aiff file, mp3 file | graph |
| Co-60 | 12 cm | aiff file, mp3 file | graph |
| Co-60 | 10 cm | aiff file, mp3 file | graph |
| Co-60 | 8 cm | aiff file, mp3 file | graph |
| Co-60 | 8 cm, again | aiff file, mp3 file | graph |
| Co-60 | 7 cm | aiff file, mp3 file | graph |
| Co-60 | 6 cm | aiff file, mp3 file | graph |
| Co-60 | 5 cm | aiff file, mp3 file | graph |
Note the general shape of the graph. Compare the intensity of the radiation at a selected distance with the intensity at twice the distance. Repeat the comparison starting with a different distance and twice the new distance.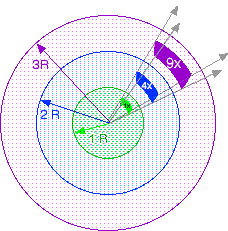
Many kinds of things that spread out in straight lines with distance (such as sunlight and sounds) without getting lost (not in muddy water or fog) decrease in intensity with distance. Imagine some energy starting at a source then spreading as an expanding sphere centered on the source. Consider the energy spread over a little patch (green) of the sphere some distance from the source (R). At twice the distance (2R) the energy will have spread over a patch (blue) twice as long and twice as wide as the original patch, that is FOUR times the area. So at twice the distance (2R), the intensity per area of the energy will be reduced to 1/4. At three times the distance (3R), the energy will be spread over NINE times the area (purple), so the intensity will be 1/9. At four times the distance (4R), the energy will be spread over SIXTEEN times the area, so the intensity will be 1/16. In general the intensity drops with the inverse of the square of the distance. Inspect your graph to determine if radiation also decreases by the inverse square of the distance: ~1/R2.
Now it is clear that one way to provide protection from potential radiation damage is to stay away from the source! At some distance away from a radioactive source the radiation spreads out so much that it's intensity has dropped until it is indistinguishable from that of background.
Communicating technical information such as observations and findings is a skill used by scientists but useful for most others. If you need course credit, use your observations in your journal to construct a formal report.
![]()
to next experiment
to nuclear chemistry menu
to ie-Chemistry menu
to site menu