

Back in England at Manchester University, Ernest Rutherford, with his assistant Hans Geiger (born 1882, died 1945) and their student Ernest Marsden (b1889, d1970), in 1909 used a radiation to bombard gold foil to investigate electron arrangements inside atoms. Expecting very small deflections by the low mass electrons, they were surprised when some α rays (about 1 in 8000) were deflected large angles. Rutherford described the result: It was quite the most incredible event that has ever happened to me in my life. It was almost as incredible as if you fired a 15-inch shell at a piece of tissue paper and it came back and hit you.
In 1911 Rutherford proposed that such a large deflection required most atomic mass to be be concentrated in a small nucleus. They proceded to use the scattering to probe the charge and size of the nucleus.
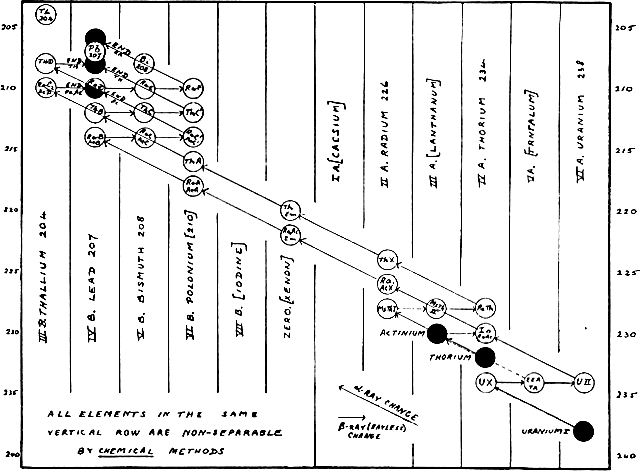
 Frederick Soddy (b1877, d1956) explained the relation of the large number of new radioactive elements to the periodic chart. Release of α-rays produces an element with atomic number 2 less and atomic weight 4 less. Release of β-rays produces an element of the same atomic weight, but with atomic number 1 greater. The new elements are not totally new kinds, but isotopes. Isotope, derived from Greek, means
Frederick Soddy (b1877, d1956) explained the relation of the large number of new radioactive elements to the periodic chart. Release of α-rays produces an element with atomic number 2 less and atomic weight 4 less. Release of β-rays produces an element of the same atomic weight, but with atomic number 1 greater. The new elements are not totally new kinds, but isotopes. Isotope, derived from Greek, means the same place.
They are atoms of the same element but having different atomic masses.
In 1913 Henry Gwyn Jeffreys Mosley (b1887, d1915, at left), working with Rutherford in Manchester, investigated the frequency of X-rays produced when cathode rays strike various metals. Mosley found the frequency (ν) of the X-rays depends on an integer which he called atomic number. Mosley interpretted the atomic number to be the electric charge on the nucleus, and the basis of the order of elements on the periodic chart. (Frequency is the quantitative equivalent to light's color. Frequency is the number of waves that pass the observer each second. It is inversely proportional to the wavelength.)


| introduction | Greeks | alchemy | Lavoisier | Dalton | Berzelius | molecules | spectra | electron | radiation | Bohr | ↑ | synthesis |
| to site menu | Discovery and Naming of Chemical Elements |
chemistry | physics | |||||||||
| created 23 March 2002 latest revision 1 May 2010 |
by D Trapp | |||||||||||