![]()
![]()
Many people find radioactivity scary. In experiment N-2 we found that radiation intensity decreases dramatically with distance. In this experiment we will investigate another way we can protect ourselves from the effects of the high energies released by radioactive events.
Radiation is emitted from the nucleus of an atom then a nuclear change occurs in that particular atom. This process seems nearly random. But it depends on the number of atoms present and the likelihood for the particular nucleus to change. Often once an atom has changed, its tendency to undergo additional change depends on the stability of the nucleus now present. In the simplest case, a radioactive atom changes to a completely stable atom. In such a case there are less radioactive atoms present so the intensity of radiation decreases with time. Even in the more complicated case where one kind of radioactive nucleus generates another radioactive nucleus, the intensity of the radiation coming for each kind of nucleus continues to depend on the number of that nucleus present.
There are several conventions used to distinguish radioactive substances:This notation is particularly useful because generally each different kind of nucleus has different properties such as the property investigated in the following experiment.
Because you may not have a Geiger counter and convenient source of radiation, a Geiger counter's electrical signal has been recorded in a sound file rather than sent to a speaker. This stored signal can be played through your computer's speakers allowing you to count the radiation as if you had been present when the experiment was recorded.
In this experiment we will investigate how the intensity of γ (gamma) radiation emitted from a small amount of 56Ba137m metal ions varies with time.
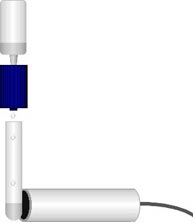
This isotope of Barium does not store well, so it generated by a nuclear reaction just before it is needed. An isotope of the metal Cesium, 55Cs137, is attached to a filter inside a minigenerator (blue in diagram). The Cesium radioactively changes to 56Ba137m by the reaction
Note this is accomplished by the spontaneous emission of a type of radiation distinguished and named β (beta) by Ernest Rutherford. The meta-stable Barium is eluted from the filter using a dilute aqueous solution of NaCl and HCl. This solution is first placed in the plastic squeeze bottle shown above the minigenerator. When the bottle is squeezed, the solution is forced through the filter in the minigenerator, drips out and is caught in the small test tube below. Immediately the intensity of the radiation is counted with the Geiger counter placed next to the test tube. The sound file has been cut into segments to make it easier to download and recount. At first the γ radiation comes too rapidly to count. To dilute the signal enough to count, it was electronically filtered inside the Geiger counter to remove 9/10 of the pulses. To find the actual radiation intensity of the first 7 minutes, calculate the original intensity by multiplying each of those counts by 10. The counts were not reduced by electronic filtering during the last 8 minutes of the experiment, or during the background count.
1. Using a clock with a second hand to keep track of the time, count the Geiger counter clicks
for EACH minute. Downloading the sound files may require patience!
| Measurement | Radiation Source | sound file | visual plot |
| minutes 1 through 3 | 56Ba137m | aiff file, mp3 file | graph |
| minutes 4 through 7 | 56Ba137m | aiff file, mp3 file | graph |
| minutes 8 through 11 | 56Ba137m | aiff file, mp3 file | graph |
| minutes 12 through 15 | 56Ba137m | aiff file, mp3 file | graph |
| two minutes | background | aiff file, mp3 file | graph |
2. Using the Geiger counter clicks
due to background radiation for the two minutes, calculate the counts per minute.
3. Construct a line graph of the corrected radiation counts verses time. Be careful to multiply the counts for the first 7 minutes by 10 before subtracting the background radiation from each measurement.
4. Experimental errors in the data make it difficult to accurately establish the shape of a graph's curve. For some phenomena that decreases like radiation, a graph using semi-log graph paper results in a straight line. Construct a line graph on semi-log graph paper of the corrected radiation counts verses time. Notice how long it takes for the radiation to decrease to half its value. Determine this time starting at several different times and take an average. This half life, t1/2, is a characteristic property of 56Ba137m. Different types of nuclei have different half lives.
There are several equations that use half life: The rate radiation is emitted is given by
where N is the number of nuclei of that type present, and R is the rate of decay (using the same time unites as the half life, t1/2).
The second equation may be used to determine N, the number of nuclei remaining after a period of time:
N is the number of nuclei present after time, t. No is the initial number of nuclei. The exponent, n, on the 1/2 is the ratio of the elapsed time divided by the half life (time units must be identical).
The second equation may be modified to determine the rate of decay after that period by substituting R for each N:
where R is the rate of decay after time, t. Ro is the initial rate. The exponent, n, on the 1/2 is the ratio of the elapsed time divided by the half life (time units must be identical).
Finally a thought about the hazard of radiation. Earlier experiments mentioned that high energy radiation is hazardous to living tissue. The previous experiment noted that one way to reduce the risk is to increase distance from the radioactive source. This experiment suggests another suggestion. Short-lived materials such as Ba-137m lose their hazard rapidly over time. Their risk can be reduced by keeping living things away from the radioactive source for a time until it spontaneously changes to a less hazardous material.
Communicating technical information such as observations and findings is a skill used by scientists but useful for most others. If you need course credit, use your data recorded in your journal to construct a formal report.
![]()
to next experiment
to nuclear chemistry menu
to ie-Chemistry menu
to site menu