![]()
![]()

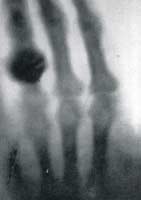 New technology often leads to a number of different discoveries. A series of discoveries flowing out of the investigations of cathode rays made possible by the development of von Geurek's vacuum pump and William Crooks evacuated tubes. in 1895 Wilhelm Konrad Röntgen (b1845, d1923 at right→) was among those investigating cathode rays. On November 8th he noted that while he was generating cathode rays inside a Crookes' tube, nearby (up to 2 m away) paper coated with barium platinocyanide lit up with brilliant fluorescence. Knowing that the cathode rays could not penetrate the glass walls of the tube, Röntgen realized some unknown radiation, which he called X-rays, must pass through glass and air to excite the nearby fluorescence crystals. The rays originated from within the glowing Crookes' tube. Röntgen stayed up all night experimenting and for a time ate and slept in the laboratory. Röntgen found X-rays exposed photographic film. He found X-rays pass through low density materials such as the soft tissue of a hand but less through materials containing elements with higher atomic weight, so that bones would cast a shadow on fluorescent materials or photographic emulsions. (at left Röntgen's first X-ray, click to enlarge) Within the next few days he demonstrated that the X-rays have some of the characteristics of high energy electromagnetic waves. (Electromagnetic radiation ranges from high energy, short wave-length gamma and X-rays, through ultraviolet light, visible light, and infrared, to low energy, low wave-length radio waves.) He considered whether X-rays could be a longitudinal wave form of electromagnetic radiation. He concluded that if X-rays are a kind of light, then they differed from the then highest energy known form of electromagnetic wave, ultraviolet light, in the following ways.
New technology often leads to a number of different discoveries. A series of discoveries flowing out of the investigations of cathode rays made possible by the development of von Geurek's vacuum pump and William Crooks evacuated tubes. in 1895 Wilhelm Konrad Röntgen (b1845, d1923 at right→) was among those investigating cathode rays. On November 8th he noted that while he was generating cathode rays inside a Crookes' tube, nearby (up to 2 m away) paper coated with barium platinocyanide lit up with brilliant fluorescence. Knowing that the cathode rays could not penetrate the glass walls of the tube, Röntgen realized some unknown radiation, which he called X-rays, must pass through glass and air to excite the nearby fluorescence crystals. The rays originated from within the glowing Crookes' tube. Röntgen stayed up all night experimenting and for a time ate and slept in the laboratory. Röntgen found X-rays exposed photographic film. He found X-rays pass through low density materials such as the soft tissue of a hand but less through materials containing elements with higher atomic weight, so that bones would cast a shadow on fluorescent materials or photographic emulsions. (at left Röntgen's first X-ray, click to enlarge) Within the next few days he demonstrated that the X-rays have some of the characteristics of high energy electromagnetic waves. (Electromagnetic radiation ranges from high energy, short wave-length gamma and X-rays, through ultraviolet light, visible light, and infrared, to low energy, low wave-length radio waves.) He considered whether X-rays could be a longitudinal wave form of electromagnetic radiation. He concluded that if X-rays are a kind of light, then they differed from the then highest energy known form of electromagnetic wave, ultraviolet light, in the following ways.
His preliminary communication
was presented before the end of the year. (A translation of the original German publication is among Carmen Guinta's classic papers.) On New Year's Day 1896, Röntgen mailed copies of his paper and photographs to a number of other scientists.
 At the weekly meeting of the French Academy of Science in Paris on the evening of January 20, 1896, Henri Poincaré describe the recent discovery of X-rays and show photographs of living bone that Röntgen had taken. Poincaré wondered out loud during his talk if X-rays were emitted by other luminescent bodies. Antoine Henri Becquerel (1852-1908 at right) undertook to investigate that hunch the next day. His father had been an authority on phosphorescence for many years. Henri himself had studied phosphorescence including that of some uranium compounds and had photography experience. It was proposed that a phosphorescent mineral would augment the cathode rays or ultraviolet light creating the X-rays. Becquerel and others presumed a substance had to luminesce in order to emit X-rays.
At the weekly meeting of the French Academy of Science in Paris on the evening of January 20, 1896, Henri Poincaré describe the recent discovery of X-rays and show photographs of living bone that Röntgen had taken. Poincaré wondered out loud during his talk if X-rays were emitted by other luminescent bodies. Antoine Henri Becquerel (1852-1908 at right) undertook to investigate that hunch the next day. His father had been an authority on phosphorescence for many years. Henri himself had studied phosphorescence including that of some uranium compounds and had photography experience. It was proposed that a phosphorescent mineral would augment the cathode rays or ultraviolet light creating the X-rays. Becquerel and others presumed a substance had to luminesce in order to emit X-rays.
Becquerel studied several different phosphorescent substances, but with no results. However he was hopeful for success with uranium compounds, which emit and absorb a whole series of harmonic luminous radiations, seem to have a particularly remarkable molecular constitution, at least from the point of view of absorption and phosphorescence.
Having loaned out his samples of uranium, he did not begin investigating phosphorescent uranium compounds for several weeks. At the February 24, 1896 meeting of the French Academy of Science, he claimed success, reporting that uranium potassium sulfate (after being made to luminesce by sunlight) emitted rays which reduced the silver bromide emulsion on a photographic plate. He had placed coins and other objects under the crystals and the shapes were reproduced on the photographic plate.
Becquerel's technique was to double wrap a photographic plate in black paper or cloth to keep out all but X-rays, tape uranium salts on top and lay the apparatus on the window sill where ultraviolet from the sun would cause the salt to fluoresce. After a prolonged exposure to ultraviolet light, he would develop the photographic plate and inspect for exposure caused by the penetrating X-rays. On February 26 and 27 Becquerel again prepared plates coated with silver bromide, wrapped in black cloth, with uranium potassium sulfate [SO4(UO)K+H2O] salts taped on top. But when it began to rain and threaten the apparatus, Becquerel suspended the experiment, placing the salt with the wrapped photographic plate underneath in the darkness of a bureau drawer to await the return of the sun. Paris remained wet and overcast several days. Becquerel developed the photographic plate on Sunday, probably hoping to find a slight exposure caused by the brief fluorescence which he might report to the French Academy of Science on Monday. Becquerel reported: I developed the photographic plates on the 1st of March, expecting to find the images very weak. Instead the silhouettes appeared with great intensity. I immediately thought that the action had to continue in darkness...
...these rays, whose effects have a great similarity to the effects produced by the rays studied by Röntgen, are invisible rays emitted by phosphorescence and persisting infinitely longer than the duration of the luminous rays emitted by these bodies. However, the present experiments, without being contrary to this hypothesis, do not warrant this conclusion.
i.e., Becquerel realized that his experiments found rays SIMILAR to X-rays, but the experiments did not establish that these rays generated without any initializing energy ARE X-rays. (Carmen Guinta translates the original French papers.) The term radiation which Becquerel meant to be generic became the specific term that is still applied to these rays.
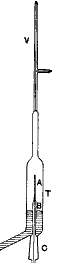
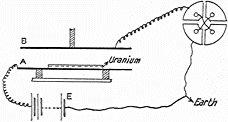 Ernest Rutherford (1871-1937) was born and raised in New Zealand but after gaining his BA, MA and BSc was awarded a scholarship at age 24 to be a student at Trinity College, Cambridge, England beginning in 1895. After studying in the Cavendish Laboratory with J.J. Thomson, Rutherford left England in 1898 for a job as Professor of Physics at McGill University, Montreal. In Canada Rutherford noted that the radiation from Uranium which caused electric charge to leak away could be blocked by covering with increasing thicknesses of Aluminum. (his apparatus is at right) But after much of the radiation was blocked, additional Aluminum reduced the remaining radiation much more gradually. In 1899 he concluded that Uranium must emit two distinct types of radiation with differing penetrating powers. Rutherford named these using the Greek alphabet α (pronounced
Ernest Rutherford (1871-1937) was born and raised in New Zealand but after gaining his BA, MA and BSc was awarded a scholarship at age 24 to be a student at Trinity College, Cambridge, England beginning in 1895. After studying in the Cavendish Laboratory with J.J. Thomson, Rutherford left England in 1898 for a job as Professor of Physics at McGill University, Montreal. In Canada Rutherford noted that the radiation from Uranium which caused electric charge to leak away could be blocked by covering with increasing thicknesses of Aluminum. (his apparatus is at right) But after much of the radiation was blocked, additional Aluminum reduced the remaining radiation much more gradually. In 1899 he concluded that Uranium must emit two distinct types of radiation with differing penetrating powers. Rutherford named these using the Greek alphabet α (pronounced alpha
), and β (pronounced beta
). (See E. Rutherford, Uranium Radiation and the Electrical Conduction, in the Philosophical Magazine, January 1899) β radiation has the characteristics of fast electrons. In 1900 the French physicist Paul Villard (1860-1934) found an even more penetrating type of radiation which was called γ (pronounced gamma
). He recognized γ has the properties of X-rays.
Ernest Rutherford and Frederick Soddy (1877-1956) suggested in 1902 that emission of radiation from radioactive materials results in the formation of new elements. (See E. Rutherford & F Soddy, The Cause and Nature of Radioactivity, in Philosophical Magazine)
 Rutherford (←photo at left) returned to New Zealand to be married then in 1907 returned to England to a job at Manchester University. In 1909 Rutherford and Thomas Royds (1884-1955) captured α radiation from radium, compressed the resulting gas above mercury flooded into the apparatus (sketched at left), and identified its spectra excited by a spark to be that of Helium. (See E. Rutherford & T. Royds, The Nature of the a Particle from Radioactive Substances, Philosophical Magazine)
Rutherford (←photo at left) returned to New Zealand to be married then in 1907 returned to England to a job at Manchester University. In 1909 Rutherford and Thomas Royds (1884-1955) captured α radiation from radium, compressed the resulting gas above mercury flooded into the apparatus (sketched at left), and identified its spectra excited by a spark to be that of Helium. (See E. Rutherford & T. Royds, The Nature of the a Particle from Radioactive Substances, Philosophical Magazine)
Albert Einstein's 1905 mathematical analysis of Brownian motion (as being due to random collisions of atoms and molecules with much larger visible particles) provided what seemed like concrete evidence that finally convinced scientists that atoms are real and not just a convenient chemical hypothesis. With the electron established to be 1/1800 the mass of the lightest atom, and having been produced from cathodes made from a large number of the elements, it seemed reasonable that all atoms contain one or more electrons. J.J. Thomson has suggested that atoms might be like a plum pudding with a positively charged dough with much smaller raisin like electrons embedded in the dough. Realizing that the positively charged α particle might serve as a useful probe to investigate if there is a random or organized electron structure inside the atom, assistant Hans Geiger and graduate student Hans Marsden undertook an experiment under Rutherford's guidance. Using an α source in a lead shielded box with only a narrow beam allowed to emerge a small hole, Geiger and Marsden aimed the beam to strike atoms. Using the thinnest material, Gold foil only a few hundred atoms thick, they expected the extremely high kinetic energy α particles to stream through the positive dough inside the Gold atoms but perhaps be slightly deflected revealing negative electron distribution in the dough.
Tracking the path of α particles was challenging. (Only later Geiger invented an electronic device to detect and count radiation.) Their procedure was to have the α particles strike a florescent screen and reveal their location. But because each flash of light was very dim, they had to sit in a darkened room for some time to adjust their eyes before counting flashes seen by observing a small patch of screen with a microscope for hours. The experiment took months of counting the number of α caused flashes at each of a number of positions representing various angular deflections. Marsden found that the great majority of the α particles passed nearly straight though the Gold atoms, being only slightly slowed and with their paths only slightly deflected.
But Geiger noticed that the number scattered more than 10° was higher than expected and about 1 in 8000 was actually deflected more than 90°, an impossibility if Thomson's plum pudding model was correct. They shared the results with Rutherford. He himself confirmed that the results were real and not due to a flawed procedure. Rutherford later said, It was almost as incredible as if you fired a fifteen inch (artillery) shell at a piece of tissue paper and it came back to hit you.
But convinced that the results were real, Rutherford pondered the implication and eventually suggested that the Gold atoms must have a structural core, what he called a nucleus, where most of the mass and positive electric charge of the atom would reside.
He sent Geiger and Marsden back to make more measurements. They were able to determine that the α particles were deflected by electrical repulsion and that the distribution of emerging α indicated that the Gold nucleus has an approximate charge of 79 elementary charges, a number equal to the Gold's atomic number. They were unable to detect any deviation from Coulomb (electrical) repulsion which suggested that no contact collision had occurred between the &alpha' particle and the tiny Gold nucleus. This implied that the nucleus was smaller than 10-14 m, less than 1/1000 of the atom radius!
So immediately the plum puddling model of the atom was rejected and some suggested an atomic model based on a miniature solar system. That atoms with orbiting electrons seemed to have a fundamental problem: Planets and moons can perpetually orbit, help in place by gravitational attraction. But the electrical attraction between the negatively charged electrons and the positively charged nucleus was a little different. Maxwell had noted that accelerated charges radiate energy as electromagnetic waves. Hertz had verified that. And radio communication made his confirmation irrefutable. So instead of a perpetual orbit, an orbiting electron would spiral in towards the nucleus, and in less than a fraction of a second, every atom so constructed would collapse! So Rutherford announced the discovery of the nucleus in 1911 but refrained from seriously proposing a new atomic model.
Two years later another of Rutherford's student's would propose a solution to the dilemma. But that proposal contained radical assumptions that many people still find unreasonable even today.
Taking a job at the Cavendish Laboratory in 1917 after J.J. Thomson's retirement, Rutherford returned to the Cavendish as Director. Under his supervision, Nobel Prizes were awarded to Chadwick for discovering the neutron (in 1932), Cockcroft and Walton for splitting the atom using a particle accelerator and Appleton for demonstrating the existence of the ionosphere. He was admitted to the Order of Merit in 1925 and in 1931 was created Baron Rutherford of Nelson of Cambridge in the County of Cambridge.
Rutherford caused the first man-made radioactive decay in 1919 by bombarding nitrogen with alpha particles, producing protons. This became the basis for all of nuclear physics that followed.
42α + 147N → 178O + 11p
C.P.Snow described Rutherford as a big, rather clumsy man, with a substantial bay-window that started in the middle of the chest. He didn't look in the least like an intellectual. But no one could have enjoyed himself more, either in creative work or the honors it brought him. He worked hard, but with immense gusto. His insight was direct, his intuition, with one exception, infallible. No scientist has made fewer mistakes. He was as original as Einstein, but he did not revolt against formal instruction.
By thirty he had set going the science of nuclear physics-single-handed, in the isolation of late-Victorian Montreal. By forty in Manchester, he had found the structure of the atom on which all modern nuclear physics depends.
Many who knew Rutherford thought him the greatest experimental scientist of the Century. Rutherford made Cambridge in the 1920s and 1930s the metropolis of experimental physics for the world.
Americans who studied there brought back Rutherford's mixture of experiment and theory that started the technological revolution that made the United States the top country in the world.
Science under the leadership of Rutherford and his peers became the greatest single force for change since the discovery of agriculture. They not only accepted this role for science, but they rejoiced in it. They viewed the scientific-technical-industrial revolution as doing incomparably more good than harm.
Rutherford himself never built the great machines of particle physics, though some of his pupils started them. Rutherford himself worked with bizarrely simple apparatus. He once proclaimed:
I could do research at the North Pole.
His researches remain the last supreme single-handed achievement in fundamental physics ...in the Cavendish phrase, with sealing wax and string.
to follow
![]()
next Experiment
to ie-Physics menu
to site menu