![]()
![]()
After Alesandro Volta (b1745, d1827) discovered in 1800 that a steady electric charge current could be produced by layering multiple sandwiches of two dissimilar metals and wet pasteboard (the electric battery), much was learned about electricity and magnetism. André-Marie Ampère (b1775, d1836), Hans Christian Oersted (b1777, d1851), Michael Faraday (b1791, d1867) and Carl Friedrich Gauss (b1777, d1855) determined the laws of electricity and magnetism which were restated and completed by James Clerk Maxwell (b1831, d1879) by 1862. The most dramatic of Maxwell's predictions, published in 1865, was that any accelerated electric charge should generate electromagnetic waves moving at the speed that Maxwell calculated should be 3 x 108 m/sec. Since this was nearly identical to the known speed of light, Maxwell concluded that light itself must be an electromagnetic wave. But by vibrating electric charges at other frequencies, it should be possible to generate other, non-visible electromagnetic waves. Frederick William Herschel (1738-1822) had already discovered infrared light in 1800 and Johann Wilhelm Ritter (1776-1810) discovered ultraviolet light the following year. But those had only slightly higher and lower frequencies than visible light.
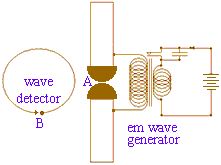
 In 1886, seven years after Maxwell's death, Heinrich Rudolf Hertz (b1857 in Hamburg, d1894, ←portrait at left) attempted to generate and detect electromagnetic waves predicted by Maxwell's theory. To generate electromagnetic waves Hertz used a high voltage induction coil to cause a spark discharge between two pieces of brass. Describing his generator, Hertz wrote
In 1886, seven years after Maxwell's death, Heinrich Rudolf Hertz (b1857 in Hamburg, d1894, ←portrait at left) attempted to generate and detect electromagnetic waves predicted by Maxwell's theory. To generate electromagnetic waves Hertz used a high voltage induction coil to cause a spark discharge between two pieces of brass. Describing his generator, Hertz wrote Imagine a cylindrical brass body, 3 cm in diameter and 26 cm long, interrupted midway along its length by a spark gap whose poles on either side are formed by spheres of 2 cm radius.
He presumed that once a spark formed a conducting path between the two brass conductors, charge would rapidly oscillate back and forth, emitting electromagnetic radiation of a wavelength about the size of his conductors. To detect the electromagnetic waves Hertz used a piece of copper wire 1 mm thick bent into a circle of diameter 7.5 cm, with a small brass sphere on one end, and the other end pointed with a screw adjustment so that the point could be moved very close to the sphere. With the detector designed so that any current flowing in the loop should match the frequency of that of his generator, he hoped to find evidence in any visible spark that jumped the tiny gap. Hertz was able to detect the electromagnetic waves up to fifty feet away. In a series of experiments he established that the electromagnetic radiation can be reflected and refracted, and that it was polarized.
 The limiting factor for detecting the radiation was the difficulty of seeing the spark in the tiny gap in his detector. When Hertz placed the detector in a darkened box in order to better see the spark, he observed that the maximum spark length was reduced by the box. He wrote
The limiting factor for detecting the radiation was the difficulty of seeing the spark in the tiny gap in his detector. When Hertz placed the detector in a darkened box in order to better see the spark, he observed that the maximum spark length was reduced by the box. He wrote I occasionally enclosed the spark B in a dark case so as to more easily make the observations; and in so doing I observed that the maximum spark-length became decidedly smaller in the case than it was before. On removing in succession the various parts of the case, it was seen that the only portion of it which exercised this prejudicial effect was that which screened the spark B from the spark A. The partition on that side exhibited this effect, not only when it was in the immediate neighbourhood of the spark B, but also when it was interposed at greater distances from B between A and B. A phenomenon so remarkable called for closer investigation.
Hertz first checked for some kind of electromagnetic effect, but found a sheet of glass effectively reduced the spark. He then found a slab of quartz did not reduce the spark. He then used a quartz prism to spread the various electromagnetic wavelengths emitted by the generator's spark. He found that the wavelength which made the little spark more powerful was ultraviolet light, much shorter than the longer waves he had designed his apparatus to generate and detect. In 1887, Hertz ended that investigation: ... I confine myself at present to communicating the results obtained, without attempting any theory respecting the manner in which the observed phenomena are brought about.
Hertz did not further investigate the effect where short wavelength ultraviolet light seemed to cause or assist the emission of the spark.
But this minor prejudicial effect
did draw the attention of others: The following year Wilhelm Hallwachs, in Dresden, wrote: In a recent publication Hertz has described investigations on the dependence of the maximum length of an induction spark on the radiation received by it from another induction spark. He proved that the phenomenon observed is an action of the ultraviolet light. No further light on the nature of the phenomenon could be obtained, because of the complicated conditions of the research in which it appeared. I have endeavored to obtain related phenomena which would occur under simpler conditions, in order to make the explanation of the phenomena easier. Success was obtained by investigating the action of the electric light on electrically charged bodies.
Hallwachs used a circular plate of Zinc with its dull oxide surface corrosion removed. He mounted the Zinc on an insulating stand and attached a wire to a gold leaf electroscope, then charged the Zinc plate negatively. The electroscope indicated that the plate lost its charge only very slowly. However, when the zinc plate was exposed to ultraviolet light from an arc lamp, or from burning Magnesium, the negative charge quickly was lost. But if the plate was positively charged, that charge was only lost slowly.
In 1899 following his measurements of cathode rays (which soon became known as electrons), J.J. Thomson investigated this ultraviolet light effect and determined that the lost electric charge was due to ejected electrons identical to those in cathode rays. Avoiding the problem of metal surface corrosion by using apparatus inside a vacuum container, Thomson utilized the basic apparatus he had previously used to investigate cathode rays. Instead of heating the metal of the cathode used to emit electrons, he illuminated the cathode with ultraviolet light. While others had little explanation of the phenomena, it was clear to Thomson that atoms in the metal cathode contained electrically charged electrons, which were caused to vibrate by the oscillating electric field of the incident ultraviolet light. Some of the electrons would be vibrated violently enough to be ejected from the metal surface.Presumably if the intensity of ultraviolet light were increased, more energy would be transferred to the electrons so more would be ejected.

 In 1902, Philipp Eduard Anton von Lenard (b1862 in Pressburg, then Austria-Hungary, d1947 show ←left & right→) used a carbon arc light which could by varied in intensity by a factor of a thousand, to study how the energy of the emitted electrons varied with the intensity of the light. He arranged his apparatus so ejected electrons would be collected by a second metal plate which was wire into a completed circuit including a sensitive ammeter to measure the current of ejected electrons. To measure the energy of the ejected electrons, Lenard attached a variable voltage source to the collector to make it more difficult for the ejected electrons. The voltage could be increased until even the electrons ejected with the most kinetic energy would be repelled from arriving at the collector. This would effectively measure the maximum energy of the electrons ejected from the metal cathode by the ultraviolet light. Lenard was surprised to find that the stopping Voltage did not depend at all on the intensity of the light. Doubling the light intensity seemed to doubled the number of electrons emitted, but did not affect the energy of each emitted electron.
In 1902, Philipp Eduard Anton von Lenard (b1862 in Pressburg, then Austria-Hungary, d1947 show ←left & right→) used a carbon arc light which could by varied in intensity by a factor of a thousand, to study how the energy of the emitted electrons varied with the intensity of the light. He arranged his apparatus so ejected electrons would be collected by a second metal plate which was wire into a completed circuit including a sensitive ammeter to measure the current of ejected electrons. To measure the energy of the ejected electrons, Lenard attached a variable voltage source to the collector to make it more difficult for the ejected electrons. The voltage could be increased until even the electrons ejected with the most kinetic energy would be repelled from arriving at the collector. This would effectively measure the maximum energy of the electrons ejected from the metal cathode by the ultraviolet light. Lenard was surprised to find that the stopping Voltage did not depend at all on the intensity of the light. Doubling the light intensity seemed to doubled the number of electrons emitted, but did not affect the energy of each emitted electron.
Lenard then used different light colors and found that the maximum energy of the ejected electrons depends on the color with shorter wavelength, higher frequency light caused electrons to be ejected with more energy.
The voltage needed to prevent any electrons from being detected is a direct measure of the energy of the most energetic of electrons: KEmax = V / qe where qe is the charge of an electron. Measuring the kinetic energy of the electrons ejected by light via the photoelectric effect led to three troubling findings:
 In 1905 Albert Einstein (1879-1955, at right→) recalled Planck's paradox and used it to explain the troubling photoelectric effect properties. He suggested that not only was light emitted and received by atoms in quanta (bundles), but that light itself exists as quanta. He wrote:
In 1905 Albert Einstein (1879-1955, at right→) recalled Planck's paradox and used it to explain the troubling photoelectric effect properties. He suggested that not only was light emitted and received by atoms in quanta (bundles), but that light itself exists as quanta. He wrote:
...according to the idea that the incident light consists of quanta with energy hν, the ejection of cathode rays by light can be understood in the following way. Energy quanta penetrate the surface layer of the body, and their energy is converted at least in part, into kinetic energy of electrons. The simplest picture is that a light quanta gives up all its energy to a single electron; we shall assume that this happens. The possibility is not to excluded, however, that electrons receive their energy only in part from the light quantum. An electron provided with kinetic energy inside the body may have lost part of its kinetic energy by the time it reaches the surface. In addition, it is to be assumed that each electron, in leaving the body, has to do an amount of work W (which is characteristic of the body). The electrons ejected directly from the surface and at right angeles to it will have the greatest velocities perpendicular to the surface. The maximum kinetic energy of such an electron isKEmax = hν – W If the body plate C is charge to a positive potential, Vstop just large enough to keep the body from losing electric charge, we must haveKEmax = hν – W = qe Vstop where qe is the magnitude of the electric charge... If the derived formula is correct, the Vstop when plotted as a function of the frequency of the incident light, should yield a straight line whose slope should be independent of the nature of the substance illuminated.
 Einstein's 1905 explanation of the photoelectric effect using quanta of light, later called photons, was the major work cited in his award of the 1921 Nobel Prize for Physics. (Einstein's proposal on relativity was considered too speculative for a Nobel.)
Einstein's 1905 explanation of the photoelectric effect using quanta of light, later called photons, was the major work cited in his award of the 1921 Nobel Prize for Physics. (Einstein's proposal on relativity was considered too speculative for a Nobel.)
The American, Robert A. Millikan (b1868, d1953, at right→), preferring the wave theory of light, thought Einstein wrong. He painstakingly developed procedures to avoid effects of surface corrosion and other contaminates in attempts over ten years, until 1916, to find fault with Einstein's explanation. He found that the photoelectric effect from reactive metals all give straight energy/frequency graph lines, all with the same slope equal to Planck's constant, h. In the end Millikan's efforts instead provided conformation of Einstein's explanation of the photoelectric effect. For this he was awarded the 1923 Nobel Prize for Physics.
to follow
Finally, record your procedures, measurements, and findings in your journal. If you need course credit, use your observations recorded in your journal to construct a formal report
![]()
next Experiment
to ie-Physics menu
to site menu