![]()
![]()
Alchemy was the product of skilled Egyptian artists who carefully guarded their trade secrets for dyeing, painting, glass making, pyrotechnics, medical drugs, mining, and metallurgy. Despite the secrecy, it was nurtured in the Museion in Alexandria and gradually developed in the tolerance of the Moslem culture. The ritualized processes for calcination, sublimation, condensation, crystallization, albification, solidifcation, and transformation involved the incantations of mysticism as much as the vocabulary of Greek elements.
Arab physicians such as Rhazes and Avicenna codified and recorded the medicine of Alchemy in the 10th and 11th Centuries in reference texts that became the standards for centuries.
Alchemy was brought back to Europe by returning Crusaders perhaps largely for its medical benefits. (Presumably the return of the Crusaders was as wildly celebrated as that of today's crusaders. [ ...as ships and aircraft return from the Iraq war of liberation,
as this was being drafted, 5/6/2003 dwt] Despite the intent of the Crusades to spread Christianity to others, the Crusades changed the homelands more than it changed the middle east! Perhaps that will be true again?) In the 16th century the physician Paracelsus wandered widely through Europe, spreading his version of alchemy with little concern for secrecy. Once alchemy became the interest of Europeans who did not need to maintain secrecy to protect their livelihood, experimentation and the sharing of findings resulted in alchemy rapidly growing beyond its theoretical foundations.
 During medieval times, air gained the reputation as an elusive, unknowable element. (Our term gas derived as a varied pronunciation of chaos, which was Greek for unknowable.) But the development of the technique of collecting airs by displacing liquids such as water and mercury from full containers immersed upside down in larger containers resulted in the production and study of numerous gases with distinct, reproducible properties. Carefully weighing and comparing initial reactants and final products often caused changes in weights that alchemy could not explain. While some people such as Robert Boyle (←portrait at left) called for the development of a new chemistry to replace the inadequate alchemy, others such as Johannes Joachim Becker and Georg Ernst Stahl attempted to modify alchemy. In the end Antoine Lavoisier and other French friends proposed a new chemistry based on a radical new concept of element. They adopted a proposal offered a century earlier by Boyle: An element is any substance that cannot be separated into several different materials. Weight change during a chemical reaction would indicated whether a substance was separating or combining. According to Lavoisier, solids, liquids and gases were actually just three states of existence which most materials would successively exhibit at increasing temperature and could be reversed by cooling.
During medieval times, air gained the reputation as an elusive, unknowable element. (Our term gas derived as a varied pronunciation of chaos, which was Greek for unknowable.) But the development of the technique of collecting airs by displacing liquids such as water and mercury from full containers immersed upside down in larger containers resulted in the production and study of numerous gases with distinct, reproducible properties. Carefully weighing and comparing initial reactants and final products often caused changes in weights that alchemy could not explain. While some people such as Robert Boyle (←portrait at left) called for the development of a new chemistry to replace the inadequate alchemy, others such as Johannes Joachim Becker and Georg Ernst Stahl attempted to modify alchemy. In the end Antoine Lavoisier and other French friends proposed a new chemistry based on a radical new concept of element. They adopted a proposal offered a century earlier by Boyle: An element is any substance that cannot be separated into several different materials. Weight change during a chemical reaction would indicated whether a substance was separating or combining. According to Lavoisier, solids, liquids and gases were actually just three states of existence which most materials would successively exhibit at increasing temperature and could be reversed by cooling.
 Lavoisier (shown right with wife Marie→), realizing the importance of vocabulary for developing and perpetuating ideas, proposed new terms supporting the new chemistry. For example, to avoid the old term
Lavoisier (shown right with wife Marie→), realizing the importance of vocabulary for developing and perpetuating ideas, proposed new terms supporting the new chemistry. For example, to avoid the old term fire
conjuring implications of alchemy, Lavoisier substituted the new term caloric.
His book Elements of Chemistry was translated to many languages and widely read. The superiority of his explanations were apparent and formed the foundation for much new research.
Alchemy adopted the Greek view that all gases were essentially the SAME element, air, perhaps each carrying small impurity amounts of other elements. But with the development of the technique of collecting gases by displacing liquid from an inverted container, very distinct properties seemed to suggest that each gas was actually a DIFFERENT substance. This interpretation supported the radical new view of the material world proposed by Laviosier.
Baking soda (NaHCO3) and vinegar (HCH3COO) react when mixed to form a gas. An enzyme in meat promotes the decomposition of hydrogen peroxide (H2O2), making a gas. And aluminum foil (Al) reacts in water with lye (NaOH) to make a gas. In this experiment we will compare the properties of the gases produced these three different ways.
You will need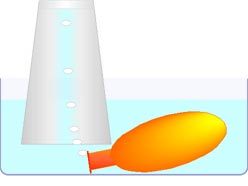
Finally, record your procedures, measurements, and findings in your journal. If you need course credit, use your observations recorded in your journal to construct a formal report
![]()
next Experiment
to ie-Physics menu
to site menu