![]()
![]()
The vast majority of the energy potentially available for use on Earth arrives in the form of visible light from the Sun. Plants have evolved to do an efficient job of capturing some of that energy and storing it as potential energy in organic molecules. Chemists have long wanted to understand this process well enough to allow the design of a molecular system to capture solar energy to meet the needs of human society. While there is sufficient solar energy to meet anticipated human needs, it has been daunting to understanding photosynthesis and using that knowledge to tailor a solution for humans.
A collection of organic chlorophyll molecules, each bonded to a central Magnesium metal ion by coordination bonds, acts as an antenna to capture light energy. The chlorophyll then transmits the energy via electrons to neighboring chlorophyll and accompanying proteins which transform the energy into a Hydrogen ion pH gradient then into more permanent chemical storage as ATP, NADP and carbohydrates. (See Expt. B9 for details.) This complicated and delicate chemical system is embedded in layered lipid membranes inside complex organelles requiring living cells for maintenance. Some chemists hope that a simpler complex of organic molecules surrounding a transition metal such as Ruthenium might provide a stable chemical system providing fuels needed to maintain human society.
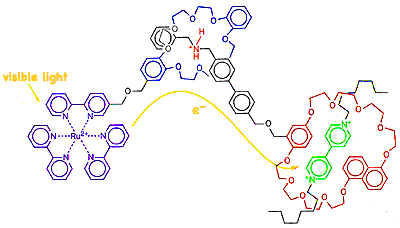 Vincenzo Balzani (U Bologna) and J. Fraser Stoddart (UCLA) appear to have developed a self assembling
Vincenzo Balzani (U Bologna) and J. Fraser Stoddart (UCLA) appear to have developed a self assembling wet
system which captures light, converts the energy to an electron which is reversibly transported away to an electron acceptor. The light capturing portion (purple) is a Ruthenium 2+ ion coordinated in a bipyridyl complex, connected to dibenzocrown ether which attaches to a central conductor section, which in turn dumps the electron into the (green) drain. Presumably these are key components necessary for eventually achieving a system which will allow the flow of photon ejected electrons to do useful work.
This self assembling approach using molecular segments which plug into appropriate matching segments seems to point to a nanochemistry revolution for assembly of new technology. While there is already a moderately well developed technology to produce electric current by photovoltaic materials, this effort to mimic photosynthesis has the potential of eventually producing low cost alternatives to petroleum. A popular first plan is to generate and collect Hydrogen gas while decomposing water to restore the system for photon capture.
Just shining visible light on an organic molecule is not sufficient to generate an electric current. While the German physicist Heinrich Hertz discovered in 1887 the photoelectric effect where electromagnetic waves caused a spark to jump a small gap in a coil of wire, that effect required either light of ultraviolet or higher energy, or an easily ionized alkali metal in a vacuum. That effect was commercially adapted for systems to automatically open doors a half century later. The current photovolteic cell is no longer an alkali in vacuum device, but rather a layered solid state junction between two dissimilar crystals of semiconductors. In both devices the flow of charge is encouraged by an applied Voltage. But in plants there is no applied voltage. So a mechanism must both encourage an electron to exit a light excited atom and to discourage the electron from simply returning to where it originated.
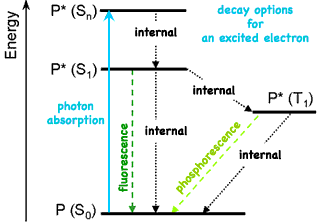
Most types of chemical bonds hold electrons relatively tightly. But metal ions have the bond disadvantage of neither having sufficient (outer) valence electrons to form covalently bonded molecules nor to have much attraction for additional electrons. The weak coordination bonds metal ions often form with pairs of electrons from electrically neutral neighbor molecules do not make for strong, stable molecules, but do provide weakly bonded electrons which serve a number of useful functions! The energy possible for bonded electrons is limited to only discrete quantized amounts. The energy levels which weakly held electrons (such as those indicated by dotted lines in diagram above) differ by only small amounts of energy. By absorbing a photon of visible light carrying energy matching that needed to be excited to a higher energy level, a weakly bonded electron can absorb that light and thereby make the material appear colored. This process of selective light absorption gives color to many of the materials we encounter in our lives. But such excited electron states are inherently unstable, so the excited electron will soon return to its normal ground state (So), typically releasing the energy as heat. But the trick used by plants is to surround the excited electron by an arrangement of atoms which, before the excited electron can return to its ground state, entice it to wander away to another location where the energy can be extracted for the plant's use.
There are several options for the unstable excited electron to release energy. Using vibrations, it can internally shift some or all the energy inside the atom (eventually releasing it as heat), simply re-emit all the energy as light, or decline to a lower permitted energy state by emitting the energy difference as light.
 The electron has some of its inherent energy/mass embedded in a quantized property (first proposed in 1925 by Ralph Kronig, George Uhlenbeck, and Samuel Goudsmit) called spin which is responsible for the electron's inherent magnetic moment, its fundamental magnetic field. (While the name originated because a spinning electrically charged body does create a magnetic field, this is inherent magnetic moment and cannot be due to actual spinning of the electron! Electrons are essential zero sized points in space, and even if a reasonable size were assigned the electron, it would need to spin faster than the speed of light to generate sufficient magnetic moment.) Based on its properties, the electron is assigned a spin of 1/2. According to the statistics of Enrico Fermi and Paul Dirac, the wave functions of such
The electron has some of its inherent energy/mass embedded in a quantized property (first proposed in 1925 by Ralph Kronig, George Uhlenbeck, and Samuel Goudsmit) called spin which is responsible for the electron's inherent magnetic moment, its fundamental magnetic field. (While the name originated because a spinning electrically charged body does create a magnetic field, this is inherent magnetic moment and cannot be due to actual spinning of the electron! Electrons are essential zero sized points in space, and even if a reasonable size were assigned the electron, it would need to spin faster than the speed of light to generate sufficient magnetic moment.) Based on its properties, the electron is assigned a spin of 1/2. According to the statistics of Enrico Fermi and Paul Dirac, the wave functions of such spin 1/2 particles
can't occupy the same space with identical particles, but can pair with the wave function of a particle of opposite spin to occupy the same space. (When then do so they are said to be in the singlet state, S, because of the single spectral line they generate, even in a magnetic field.) But while an excited electron is unpaired, it can flip to an opposite spin so that its spin is now identical to its original partner. (They are said to be in the triplet state, T, because in a magnetic field their three closely spaced but distinct energy levels produce three hyperfine spectral lines.) But once in this situation, the excited electron cannot rapidly emit its excess energy because that would place two identical electrons in the same space. So only slower processes involving spin reversal are permitted. (Spin is describes as conserved. A spontaneous spin change is forbidden unless coupled with another spin conserving change. At excited energies the probability of violating a forbidden transition is no longer absolutely zero, but remains tiny and rare.)
Each energy releasing decay is characterized by its own rate constant and each excited state is characterized by its lifetime τ. Because sometimes there is a pronounced delay of emission of light from electrons triplet state, that process was given a distinguishing name long before the process was understood. The rapid (~10 ns) re-emission of light when spin is not changed is called fluorescence while the delayed (ms to hours) release accompanying a change in spin is called phosphorescence. If the lifetime of the excited state is long enough, the excited molecule may have time to react with a neighboring molecule. Only those excited states that have a lifetime longer than ~10-9 s allow the possibility of a chemical reaction. For transition metal complexes, only the lowest spin-forbidden excited state last long enough for chemical reaction to transfer the excited electron to a neighbor molecule. Because Ruthenium 2+ ions provide an optimum long-lived excited state to maximize conversion of the absorbed photon's energy to chemical energy, they may be ideal for artificial photosynthesis.
an experiment is needed
![]()
to Nanochemistry menu
to e-Chemistry menu
to site menu