![]()
![]()
 In 1977 an ocean floor investigation off the coast of Ecuador along the East Pacific Rise discovered hot water being ejected from tall natural rock chimneys. While the deep ocean water was very cold, the ejected water was over 350°C, a temperature well above the normal 100°C boiling point for water, but kept from boiling by the immense pressure at depths averaging 7000 feet. The water carried large amounts of minerals saturated at even higher temperatures and now precipitating as the water cooled, making black smoke-like clouds.
In 1977 an ocean floor investigation off the coast of Ecuador along the East Pacific Rise discovered hot water being ejected from tall natural rock chimneys. While the deep ocean water was very cold, the ejected water was over 350°C, a temperature well above the normal 100°C boiling point for water, but kept from boiling by the immense pressure at depths averaging 7000 feet. The water carried large amounts of minerals saturated at even higher temperatures and now precipitating as the water cooled, making black smoke-like clouds.
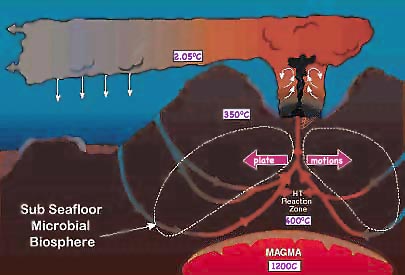 Most such hydrothermal vents located at the boundaries between the plates of the earth's crust contain high concentrations of H2S and FeS, the latter responsible for the black precipitate. These solutes cause the solution to be highly acidic. It is believed that ocean water seeps into the spreading earth's crust near the mid-ocean ridge. After heating it absorbs minerals in the crust and rises through cracks in the earth's crust due to the solution's reduced density when hotter. As the water releases heat to the surrounding ocean, cooling to the near freezing seawater common found at great depths, the dissolved minerals crystalize. They often grow chimneys above the vents many feet a year until they becomes unstable and collapses. These may reach heights of 20 meters before crumbling. Nearby considerable sediment collects on the ocean floor.
Most such hydrothermal vents located at the boundaries between the plates of the earth's crust contain high concentrations of H2S and FeS, the latter responsible for the black precipitate. These solutes cause the solution to be highly acidic. It is believed that ocean water seeps into the spreading earth's crust near the mid-ocean ridge. After heating it absorbs minerals in the crust and rises through cracks in the earth's crust due to the solution's reduced density when hotter. As the water releases heat to the surrounding ocean, cooling to the near freezing seawater common found at great depths, the dissolved minerals crystalize. They often grow chimneys above the vents many feet a year until they becomes unstable and collapses. These may reach heights of 20 meters before crumbling. Nearby considerable sediment collects on the ocean floor.
In December 2000 a hydrothermal field was found 15 km east of the Mid-Atlantic Ridge on crust 1.5 x 106 years old. The venting solution here is much cooler, between 28
Along the ocean floor an order of magnitude deeper than the maximum depth reached by even the dimmest sunlight, no life was anticipated. But surprisingly, life was discovered to be abundant in the warm water and chemical conditions toxic to most earthly life. While all previously known life depends on energy captured from the Sun by photosynthesis, apparently bacteria in and around these hydrothermal vents extract energy from the normally poisonous H2S. These are in turn eaten by giant tube worms, clams, and shrimp.
At the high temperatures in the mantle of the earth, weaker chemical bonds are broken. Some of the compounds and elements crystalize when allowed to cool and may eventually become available to surface chemistry due to erosion. Some of the magna is released to the surface in volcanic eruptions or at flows along the plate boundaries. Cooling more rapidly, this forms small crystals such as in granite and frozen solutions such as basalt. Such material as reaches the surface is available for solution in water or oxidation by the atmosphere.
The earth's crust is made up of about 80 elements, in combinations of over 2000 different compounds and minerals. Most of the crustal material is made up of six metals, Aluminum (8% Al), Iron (5% Fe), Calcium (3.6% Ca), Sodium (2.8% Na), Potassium (2.6% K) and Magnesium (2.1% Mg) combined with Oxygen (47% O), Silicon (28% Si), and Sulfur (S). These six metal elements are moderately to highly reactive as elements so usually exist combined with other elements. The elements near the middle of the periodic chart form silicates, aluminosilicates, feldspars and clay minerals. These and many compounds of the less abundant transition elements have covalent-like bonds which exhibit low water solubilities. In general, the higher the temperature of crystallization under pressure inside the earth, the less stable these minerals are at the low temperature found near the Earth's surface.
In contrast, some of the compounds of the alkalis (particularly Na, and K) and alkaline earths (Mg and Ca) are more ionic and thus have moderate solubilities in water. They and materials containing them are susceptible to dissolving and chemical weathering reactions with water and the weak carbonic acid formed when atmospheric CO2 dissolves in the water:As a consequence, few of the materials found in a kitchen or elsewhere around one's home have moderate solubilities suitable for the following investigation. The solubilities of some water soluble compounds have little temperature dependance while others change markedly with water temperature.
Communicating technical information such as observations and findings is a skill used by scientists but useful for most others. If you need course credit, use your observations in your journal to construct a formal report.
![]()
to next investigation
to Environmental Chemistry menu
to ie-Chemistry menu
to site menu