Prolog: As an early attempt to provide free, systematic, effective chemistry instruction via the Web, the following web pages present one of the newest and most fascinating parts of chemistry: biochemistry, a topic which previously had few available educational resources. Instead of presenting an enormous encyclopedia of known biochemistry, the approach is to describe the nervous system which provides us with a way to understand ourselves, how we came to be, and gives clues to what we might attain.
All known life is based on combinations of the Carbon atom (symbol: C) with itself and other kinds of atoms. Carbon atoms are optimum for this role since it is plentiful on Earth, has an intermediate electronegativity, and with four valence electrons, can form strong covalent bonds with multiple other atoms. This allows the formation of a large number of molecules ranging from tiny to enormous size, with a multitude of diverse properties providing mechanisms for all the functions necessary for life. Life reproduces, repairs, dies and recycles essential building materials. Some forms collect energy while others are mobile and gather what they need. An evolution mechanism enhanced by sèxûal reproduction provides a means for adaptation to slowly changing environmental conditions. Nervous systems provide both a way to respond to immediate needs and changes in environment, and as in humans, even a way to understand our own existence.
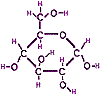 Carbohydrates are molecules with the generic formula Cn(H2O)m. (In the simplest m = n, while removal of water accompanies formation of larger molecules, so m < n.) Nearly all start as the product of photosynthesis, but can serve a variety of functions from energy storage (e.g., glucose shown at right →, glycogen, starch) to structural support (e.g., cellulose). While the molecular formula suggests that carbohydrates contain water, the Oxygen are instead attached to the sides of a chain of Carbon atoms either as hydroxyl (-OH) or carbonyl (C=O) functional groups. These get their name because a molecule functions (chemically reacts) differently than a molecule without that particular functional group. Most chemical reactions in living organisms involve these functional groups. Typically the smallest carbohydrates (generically called monosaccharides) curl into ring shapes (glucose being an example) which can be further assembled into pairs (disaccharides) and long chains (polysaccharides). The rings are added one at a time into a chain by removing a hydroxyl group (-OH) from one ring, R, and a Hydrogen atom, H, from a second ring, R'. Note water is a removed byproduct.
Carbohydrates are molecules with the generic formula Cn(H2O)m. (In the simplest m = n, while removal of water accompanies formation of larger molecules, so m < n.) Nearly all start as the product of photosynthesis, but can serve a variety of functions from energy storage (e.g., glucose shown at right →, glycogen, starch) to structural support (e.g., cellulose). While the molecular formula suggests that carbohydrates contain water, the Oxygen are instead attached to the sides of a chain of Carbon atoms either as hydroxyl (-OH) or carbonyl (C=O) functional groups. These get their name because a molecule functions (chemically reacts) differently than a molecule without that particular functional group. Most chemical reactions in living organisms involve these functional groups. Typically the smallest carbohydrates (generically called monosaccharides) curl into ring shapes (glucose being an example) which can be further assembled into pairs (disaccharides) and long chains (polysaccharides). The rings are added one at a time into a chain by removing a hydroxyl group (-OH) from one ring, R, and a Hydrogen atom, H, from a second ring, R'. Note water is a removed byproduct.
Lipids are oils and fats, generally converted from carbohydrates by removal of much of the Oxygen (and reassembly, two carbons at a time), making them a more concentrated storage forms of energy. Lipids also serve other functions from electrical insulations surrounding nerves (myelin) and water insoluble cell membranes, to control messengers (e.g., hormones such as testosterone).
Nucleic acids, in addition containing both Nitrogen atoms and Phosphate groups (-PO4), serve as reproduction and information storage (DNA = deoxyribonucleic acid), messenger and assembly information roles (RNA = ribonucleic acid) and energy transport (ATP = adenosine triphosphate).
Proteins serve as structural components (hair), control (protein gateways in cell membranes and hormones such as insulin) and as biological catalysts causing nearly all the life enabling chemical reactions which would otherwise rarely occur at the temperature of living organisms. Proteins are long tangled chain-like molecules assembled from smaller molecules called amino acids which contain at least one amine functional group (-NH2) and at least one carboxyl acid group (-COOH). The amino acids are coupled together into a chain by removing a hydroxyl group (-OH) from the carboxyl group of one amino acid and a Hydrogen atom, H, from the amine group of a second amino acid. (Here the R and R' are the rest of the molecules, and need not be the same.) Note water is again a byproduct.
(You might have noted that chemists sometimes use molecular formulae such as H2O which just give the atom counts, while at other times it is more informative to present structural formulae which attempt to show how the atoms are arranged and sometimes give clue for how they occupy space... as fainter symbols suggest further away positions.)
This might be a good time to recall that generally chemical reactions occur when molecules collide while jostling around due to their random kinetic energies which we measure as temperature. When warmer, the molecules move on average faster so get to collide sooner. So reactions should be proportionately faster at higher temperatures. But often molecules collide at weird alignments so that they merely rebound rather than break bonds allowing formation of new ones. Higher temperatures make the speeds of collision faster so also increases the chance of breaking bonds and improves chances for reaction to occur. Conversely, there is often a temperature low enough so that no collision breaks any bonds, all molecules only rebound, and the chemical reaction rate is zero. Just above this temperature reaction rates seem to jump exponentially with an increase in temperature.
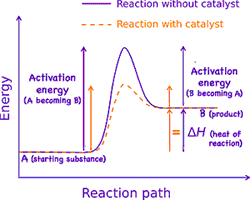 One problem facing life is that, at temperatures cool enough for life to continue to exist, nearly all of the involved chemical bonds are strong enough that reactions essentially never occur. This keeps the environment's random kinetic energy from destroying life. But life requires some change! Fortunately there are a few substances which seem to have the strange ability to promote chemical reactions. Swedish chemist Jöns Jakob Berzelius coined the phrase catalysed processes in 1836 to describe reactions which are mysteriously accelerated by substances which remain unchanged after the reaction. In the 1880s Wilhelm Ostwald at Leipzig University started systematically investigating such reactions, receiving the 1909 Nobel Prize in Chemistry for his work. Such catalysts work by providing an alternative reaction mechanism which requires a lower activation energy than that needed for reaction by collision. The effect is that more molecular collisions have the (reduced) energy needed to react. Hence, catalysts assist reactions to occur much faster, or at lower temperatures than otherwise possible.
One problem facing life is that, at temperatures cool enough for life to continue to exist, nearly all of the involved chemical bonds are strong enough that reactions essentially never occur. This keeps the environment's random kinetic energy from destroying life. But life requires some change! Fortunately there are a few substances which seem to have the strange ability to promote chemical reactions. Swedish chemist Jöns Jakob Berzelius coined the phrase catalysed processes in 1836 to describe reactions which are mysteriously accelerated by substances which remain unchanged after the reaction. In the 1880s Wilhelm Ostwald at Leipzig University started systematically investigating such reactions, receiving the 1909 Nobel Prize in Chemistry for his work. Such catalysts work by providing an alternative reaction mechanism which requires a lower activation energy than that needed for reaction by collision. The effect is that more molecular collisions have the (reduced) energy needed to react. Hence, catalysts assist reactions to occur much faster, or at lower temperatures than otherwise possible.
In living creatures, proteins often serve as catalysts. Usually proteins are loosely help into specific shapes by weak chemical bonds between adjacent functional groups. These weak bonds allow a protein to morph between alternate shapes. This controlled flexibility could, for example, allow a particular protein to encircle another molecule, use suitably placed functional groups to promote a chemical change in the encircled molecule, then release that changed molecule much as robot arms make changes in automated manufacturing factories. Such protein catalysts are called enzymes.
The following experiment involves two different kinds of protein. First protein extracted from connecting tissue is dissolved in hot water forming a solution. When the solution is cooled and allowed to sit undisturbed, functional groups of neighboring proteins form weak bonds forming a semi-solid network of molecules. This traps the much smaller water (and any added sugar, flavoring or coloring molecules) forming a gel.
Some tropical fruits contain an abundance of an enzyme which digests the strong bonds between the amino acids chained together. This may be a defense mechanism that such plants have evolved to discourage insects which might otherwise burrow into and consume the fruits packed with nutrients. The experiment uses commercially available meat tenderizer which contains papain, an enzyme abundant in fresh pineapple and papaya. The papain reverses the formation reaction shown above, consuming water, and attaching hydroxyl and hydrogen to the original locations. This a a common chemical reaction in living organisms, generically called hydrolysis, but in this case more commonly called the digestion of the protein. The same process occurs when the papain is added to the protein in tough meat. In our stomachs another enzyme, pepsin, does a similar hydrolysis! Other digestive enzymes hydrolyze glycogen, disaccharides, lipids, and nucleic acids. Most animals have difficulty digesting cellulose, but Termites culture symbiotic bacteria which produce the needed enzymes. And grazing animals have their own method using multiple stomachs.
Communicating technical information such as observations and findings is a skill used by scientists but useful for most others. If you need course credit, use your observations in your journal to construct a formal report.
![]()
If you choose next investigation,
the next screen will present information to help understand our fabulous nervous system, starting with our senses which provide us our only knowledge.... and in successive screens, explanation of how we process that information, enjoy it, remember it, and use it to think! Wow!
to next investigation
to Biochemistry menu
to ie-Chemistry menu
to site menu