![]()
![]()
Mendeléeff searched for an organizing principle so that the encyclopedia of chemical knowledge would not be chaotic. While the law of definite chemical compounds had persuasively shown the usefulness of atomic theory, a whole group of so-called indefinite compounds and solutions seemed to refute the atomic theory. Distinct chemical elements with well defined atomic weights clearly existed, but the actual existence and nature of atoms was more problematic. There was no direct evidence that atoms actually exist. On February 17, 1869 Dmitri Mendeléeff (b1834, d1907) completed his first periodic table which in March he presented in a paper to the Russian Chemical Society. In this paper he presented a periodic table, boldly noted the periodic relationship of chemical formulas to atomic weights, noted some elements had atomic weights that needed correction, and forecast that new elements would be discovered to fit vacant spaces in his table. Mendeléeff had found the weight of elements (based on their combining ratios) was an invariable characteristic that provided the desired organization for studying chemistry, an approach which avoided the existence of atoms which was still shrouded in controversy.
The establishment of the periodic system was regarded by most chemists as a discovery of the highest importance. Stanislao Cannizzaro had provided the key to establishing atomic weights with Avogadro's hypothesis. A number of chemists had found patterns in those weights. Mendeléeff had recognized the periodic table's broad power to organize all that was know about the chemical elements and their compounds plus the value for predicting yet undiscovered substances and their properties. Julius Lothar Meyer (b1830, d1895) had first used a similar pattern to merely suggest the difficulty with simple atoms but quickly appreciated and popularized the broad significance suggested by Mendeléeff. Alexandre Emile Béguyer de Chancourtois (b1820, d1886), professor of geology at the École des Mines in Paris, described to the French Academy of Sciences in 1882-3 his vis Tellurique (earthy screw) plotting atomic weights on a helix with 16 atomic mass units per turn. (However Chancourtois' screw does NOT align any chemical families; he found no chemical periodicity.) William Odling (b1829, d1921), a reader in chemistry at St. Bartholomew's Hospital in London had suggested an arithmetical seriation of atomic weights in 1864. Gustavus Detlef Hinrichs (b1836, d1923), professor at the University of Iowa, published in 1867 a radial periodic chart with each family arranged along spokes with the monomer of all atoms, an Urstoff called pantogen with a weight of half hydrogen's at the center. (But only non-metals had arcs of similar atomic weights.) It was Mendeléeff's broad appreciation for the significance and practical use of the periodic law for all elements that won him acknowledgment as its discoverer.
While the periodic table used relative weights of elements based on combining ratios in chemical compounds, it added strong support for the assumption of atoms and atomic weights. The periodic reoccurrence of similar chemical properties among elements listed by atomic weight seem to suggest that there must be some underlying structure to all matter.
 It had been long known that substances energized in flames or by electric spark emitted colors often characteristic of the elements within. Some elements have relatively simple color spectra while others such as Iron have thousands of visible, distinct colors. In 1823 the British astronomer John Herschel suggested that gases might be identified by their unique spectra of colors. Gustav Robert Kirchhoff (b1824 in Königsberg, Prussia, d1887, photo at right→) and Robert Wilhelm Bunsen (b1811 in Gottingen, d1899) invented a spectroscope to systematically study the color of elements in flames. In 1859, they found each element has spectral lines not affected by the presence of other elements. Their spectroscope, combining a prism with a magnifying telescope, separated the different colors of light so their wavelengths and intensities could be accurately measured. Spectral analysis proved to be a very productive tool for discovering elements present in only trace amounts. The spectroscope accelerated the discovery of both very reactive and thus difficult to separate elements as well as the non-reactive rare earths.
It had been long known that substances energized in flames or by electric spark emitted colors often characteristic of the elements within. Some elements have relatively simple color spectra while others such as Iron have thousands of visible, distinct colors. In 1823 the British astronomer John Herschel suggested that gases might be identified by their unique spectra of colors. Gustav Robert Kirchhoff (b1824 in Königsberg, Prussia, d1887, photo at right→) and Robert Wilhelm Bunsen (b1811 in Gottingen, d1899) invented a spectroscope to systematically study the color of elements in flames. In 1859, they found each element has spectral lines not affected by the presence of other elements. Their spectroscope, combining a prism with a magnifying telescope, separated the different colors of light so their wavelengths and intensities could be accurately measured. Spectral analysis proved to be a very productive tool for discovering elements present in only trace amounts. The spectroscope accelerated the discovery of both very reactive and thus difficult to separate elements as well as the non-reactive rare earths.

Earlier in 1802 the Englishman, William Wollaston, had noticed a several dark regions in spectra of stars. A dozen years later a superb German glassmaker, Joseph von Fraunhofer (←shown at left; b1787, died young in 1826 as many glassmakers, poisoned by vapors of heavy metals), used his own lenses and diffraction grating to measure 574 such dark lines in the spectra of the Sun. Kirchhoff noted that several of Fraunhofer's dark lines matched the two brightest lines in the spectra of Sodium. He was able to reproduce many of the Fraunhofer lines by passing a continuous spectra (from a hot wire) through relatively cool samples of gases. Apparently part of the light from the hot glowing cores of stars is absorbed by cooler outer layers of gas, providing evidence that stars are composed of the same elements as we have here on Earth.
 For nearly a century it had been known that electrical current could be produced by certain types of chemical reactions (now called oxidation-reduction reactions). While investigating the visible passage of a high voltage electric spark through near vacuum inside a glass tube in November 8, 1895, Wilhelm Konrad Röntgen (b1845, d1923, at right→) by chance noticed that nearby (up to 2 m away) a piece of paper coated with barium platinocyanide glowed. Knowing that the spark itself could not penetrate the glass and cause the glow, Röntgen realized that the glow must be the result of some unknown radiation, which he temporarily called X-rays. He found that this radiation penetrates glass, air and other low density materials such as the soft tissue of a hand but is adsorbed by elements with higher atomic weight such as the Calcium in bones. The X-rays could also be detected by exposing photographic film (containing Silver). In the next few days of investigations, Röntgen found that X-rays had the characteristics expected of high energy electromagnetic waves.
For nearly a century it had been known that electrical current could be produced by certain types of chemical reactions (now called oxidation-reduction reactions). While investigating the visible passage of a high voltage electric spark through near vacuum inside a glass tube in November 8, 1895, Wilhelm Konrad Röntgen (b1845, d1923, at right→) by chance noticed that nearby (up to 2 m away) a piece of paper coated with barium platinocyanide glowed. Knowing that the spark itself could not penetrate the glass and cause the glow, Röntgen realized that the glow must be the result of some unknown radiation, which he temporarily called X-rays. He found that this radiation penetrates glass, air and other low density materials such as the soft tissue of a hand but is adsorbed by elements with higher atomic weight such as the Calcium in bones. The X-rays could also be detected by exposing photographic film (containing Silver). In the next few days of investigations, Röntgen found that X-rays had the characteristics expected of high energy electromagnetic waves.
A few weeks later at the weekly meeting of the French Academy of Science, Henri Poincar described Röntgen recent discovery of X-rays and showed X-ray photographs of bone in a living hand. Poincaré wondered whether X-rays might also be emitted when other substances luminesce. Antoine Henri Becquerel (b1852, d1908; at left→) undertook to investigate that hunch the next day. Presuming a substance might only emit X-rays while it simultaneously emitted visible light, Henri proposed that phosphorescent minerals might also create X-rays while fluorescing. But after experimenting with several different phosphorescent minerals, Becquerel reported he had found no evidence of any X-rays. An investigation of phosphorescent Uranium compounds was delayed until Becquerel could retrieve samples on loan to friends.  Becquerel's procedure was to double wrap a primitive photographic plate in black paper or cloth to block all but penetrating X-rays, tape the mineral on top and lay the combination out on his window sill where ultraviolet from the sun would cause the mineral to fluoresce, hopefully also producing X-rays. Rain in late February caused Becquerel to suspended experimentation by placing the wrapped photographic plate with accompanying Uranium salt in the darkness of a drawer awaiting the next sunny day. When Paris remained wet and overcast for several days, Becquerel developed the photographic plate on Sunday anticipating reporting to the French Academy of Science on Monday. Becquerel reported:
Becquerel's procedure was to double wrap a primitive photographic plate in black paper or cloth to block all but penetrating X-rays, tape the mineral on top and lay the combination out on his window sill where ultraviolet from the sun would cause the mineral to fluoresce, hopefully also producing X-rays. Rain in late February caused Becquerel to suspended experimentation by placing the wrapped photographic plate with accompanying Uranium salt in the darkness of a drawer awaiting the next sunny day. When Paris remained wet and overcast for several days, Becquerel developed the photographic plate on Sunday anticipating reporting to the French Academy of Science on Monday. Becquerel reported: I developed the photographic plates on the 1st of March, expecting to find the images very weak. Instead the silhouettes appeared with great intensity. I immediately thought that the action had to continue in darkness... ...these rays, whose effects have a great similarity to the effects produced by the rays studied by Röntgen, are invisible rays emitted by phosphorescence and persisting infinitely longer than the duration of the luminous rays emitted by these bodies. However, the present experiments, without being contrary to this hypothesis, do not warrant this conclusion.
i.e., Becquerel realized that his experiments found rays similar to X-rays, but the experiments did not establish that these rays, created without any initializing energy, ARE X-rays. The term radiation which Becquerel meant to be generic became the specific term that is still applied to these rays. These two nearly accidental, serendipitous events occurred only a few months apart, and provided the break through tools to both unlock the structure of the atom, and remove all reasonable doubt as to the existence of atoms.
 By 1897 Joseph John Thomson (←shown at left; b1856, d1940) at Cambridge, found a way to remove residual gases inside a glass tube by baking so he could accomplish what others had expected but failed to do, deflect sparks inside a tube using an electric field. Using both an electric field and a countering magnetic field, Thomson was able to adjust the fields so the two forces just cancelled, leaving the spark to travel as if both fields were off. Then using the equations for both electric and magnetic force, Thomson calculated the speed of the rays, and the ratio of their electric charge to mass (q/m). The speed was near that of light. The q/m ratio was consistent with a mass less than 1/1000 that of the lightest atom, Hydrogen. Based on these calculations, Thomson proposed that electricity was carried by a tiny constituent of atoms He suggested the name electric corpuscle, but electron proposed by Goldstein became the popular label. J.J. Thomson showed that identical electrons were obtained from every kind of metal electrode he tried. Thomson concluded the electron is probably a fundamental part of all atoms.
By 1897 Joseph John Thomson (←shown at left; b1856, d1940) at Cambridge, found a way to remove residual gases inside a glass tube by baking so he could accomplish what others had expected but failed to do, deflect sparks inside a tube using an electric field. Using both an electric field and a countering magnetic field, Thomson was able to adjust the fields so the two forces just cancelled, leaving the spark to travel as if both fields were off. Then using the equations for both electric and magnetic force, Thomson calculated the speed of the rays, and the ratio of their electric charge to mass (q/m). The speed was near that of light. The q/m ratio was consistent with a mass less than 1/1000 that of the lightest atom, Hydrogen. Based on these calculations, Thomson proposed that electricity was carried by a tiny constituent of atoms He suggested the name electric corpuscle, but electron proposed by Goldstein became the popular label. J.J. Thomson showed that identical electrons were obtained from every kind of metal electrode he tried. Thomson concluded the electron is probably a fundamental part of all atoms.
 Having distinguished several types of radioactivity (α, β and γ, with only γ being electromagnetic radiation like X-rays), in 1909 Ernest Rutherford (b1871, d1937, perhaps the century's greatest experimentalist, shown at right→), with his assistant Hans Geiger (b1882, d1945) and their student Ernest Marsden (b1889, d1970), used α radiation to bombard gold foil to investigate electron arrangements inside atoms. Expecting very small deflections by the low mass electrons, they were surprised when some α rays (about 1 in 8000) were deflected large angles. Rutherford described the result:
Having distinguished several types of radioactivity (α, β and γ, with only γ being electromagnetic radiation like X-rays), in 1909 Ernest Rutherford (b1871, d1937, perhaps the century's greatest experimentalist, shown at right→), with his assistant Hans Geiger (b1882, d1945) and their student Ernest Marsden (b1889, d1970), used α radiation to bombard gold foil to investigate electron arrangements inside atoms. Expecting very small deflections by the low mass electrons, they were surprised when some α rays (about 1 in 8000) were deflected large angles. Rutherford described the result: It was quite the most incredible event that has ever happened to me in my life. It was almost as incredible as if you fired a 15-inch shell at a piece of tissue paper and it came back and hit you.
In 1911 Rutherford proposed that such a large deflection required most atomic mass to be be concentrated in a small nucleus of positive electric charge. They preceded to use the scattering to the measure the charge (79 times larger than the negative charge of an electron) and probe the size of the nucleus.
 In 1913 Henry Gwyn Jeffreys Mosley (←shown at left; b1887, died 1915 tragically fighting for the British at Tripoli in WWI), investigated the frequency of X-rays produced sparks strike various metals. Mosley found the frequency (ν) of the X-rays depends on an integer which he called atomic number. Mosley interpreted the atomic number to be the electric charge on the nucleus of the atoms in the metal, and the basis of the order of elements on the periodic chart. (Frequency is another quantitative equivalent to light's color. Frequency is the number of waves that pass the observer each second. It is inversely proportional to the light's wavelength, λ by the equation
In 1913 Henry Gwyn Jeffreys Mosley (←shown at left; b1887, died 1915 tragically fighting for the British at Tripoli in WWI), investigated the frequency of X-rays produced sparks strike various metals. Mosley found the frequency (ν) of the X-rays depends on an integer which he called atomic number. Mosley interpreted the atomic number to be the electric charge on the nucleus of the atoms in the metal, and the basis of the order of elements on the periodic chart. (Frequency is another quantitative equivalent to light's color. Frequency is the number of waves that pass the observer each second. It is inversely proportional to the light's wavelength, λ by the equation
 Rutherford created a dilemma when he proposed that each atom has a nucleus containing nearly all the atom's mass and a positive charge equal the atomic number. If an atom's negative electrons were stationed in the remaining atomic space, the electrical attraction would pull the electrons toward the nucleus, collapsing the atom. Alternatively, if the electrons were placed in motion orbiting the nucleus, the electromagnetic laws governing accelerated electric charges would require that each electron broadcast away its energy resulting in a spiral crash into the nucleus. Neither stationary nor moving electrons could form stable atoms. In 1913, Niels Bohr (b1885, d1962, shown at right→), after working with Rutherford, proposed that electrons did orbit the nucleus but that the electromagnetic laws somehow acted differently inside atoms. Bohr proposed that perhaps the angular momentum of each orbiting electron was specified by an integer (quanta) multiple of Planck's constant. Since electrons would not be permitted to have fractional quanta, an electron could not gradually broadcast.
Rutherford created a dilemma when he proposed that each atom has a nucleus containing nearly all the atom's mass and a positive charge equal the atomic number. If an atom's negative electrons were stationed in the remaining atomic space, the electrical attraction would pull the electrons toward the nucleus, collapsing the atom. Alternatively, if the electrons were placed in motion orbiting the nucleus, the electromagnetic laws governing accelerated electric charges would require that each electron broadcast away its energy resulting in a spiral crash into the nucleus. Neither stationary nor moving electrons could form stable atoms. In 1913, Niels Bohr (b1885, d1962, shown at right→), after working with Rutherford, proposed that electrons did orbit the nucleus but that the electromagnetic laws somehow acted differently inside atoms. Bohr proposed that perhaps the angular momentum of each orbiting electron was specified by an integer (quanta) multiple of Planck's constant. Since electrons would not be permitted to have fractional quanta, an electron could not gradually broadcast.
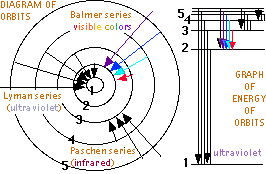
But Bohr proposed that an electron could absorb the correctly sized quanta of energy to be excited to any higher orbit allowed by a larger integer. Then the excited electron might spontaneously returning to a lower orbit, emitting the discrete color of light specified by the difference in energy of the two electron orbits. Bohr calculated the orbits possible, the energies a electron would have in each orbit, and the spectra colors for each orbit transition in the simplest atom, Hydrogen. He found a perfect match with the known spectra of Hydrogen. Noting that the periodic table has inert elements periodically, with elements that easily lose electrons on one side, and atoms that take electrons on the other side, Bohr proposed that filling or emptying an orbit that holds a specified maximum number of electrons could explain the periodic table. This proposal made it possible to successfully predict where other missing elements might be discovered.
With the short 18 years, the structure of matter that had been suspected for a century was unravelled. Atoms contained a nuclear core composed of perhaps two kinds of massive particles, one with positive electric charge and likely another with no charge. Most of the volume of the atom would be composed of very light but layered electrons.
In the years that followed, the proton and neutron were identified as constituents of the nucleus. Max Planck and Albert Einstein had already pointed out that light could paradoxically be thought of as both particles and waves. And soon Louis deBroglie would suggest that matter has similar strange properties, most noticeable at the tiny scale of atoms. So Bohr's orbits of particle-like electrons were abandoned in exchange for thinking of electrons as clouds with quantized energies, simultaneously occupying entire regions of space around the nucleus. The resulting quantum mechanics turned out to be one of the most successful scientific theories of all time.
There is an important difference between chance discovery and the serendipity often involved in scientific discoveries. Serendipity typically occurs when people have (1) trained and prepared themselves to understand both what existing theories predict should happen, and (2) recognize when something unexpected occurs. Accidents generally are thought to occur with no preparation necessary.
![]()
to menu for Introductory Chemistry
to ie-Chemistry menu
to site menu