![]()
![]()
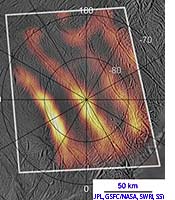 Enceladus is Saturn's 6th largest moon, with a small diameter of 500 km. It orbits in the densest part of Saturn's diffuse E ring. Its surface is primarily covered with ice (H2Os) which reflects nearly 100% of the sunlight. Observations in 2005 suggested that cracks in Enceladus' southern hemisphere released plumes of ice and water vapor. A heat map (←) made from data collected in 2008 suggest that the cracks have temperatures as warm as 180 K (-93°C) compared to a general surface temperature averaging 75 K. Ultraviolet spectrograph data suggests that the plume is mostly gaseous water, with methane, carbon monoxide, carbon dioxide, and a mix of both simple and complex organic molecules.
Enceladus is Saturn's 6th largest moon, with a small diameter of 500 km. It orbits in the densest part of Saturn's diffuse E ring. Its surface is primarily covered with ice (H2Os) which reflects nearly 100% of the sunlight. Observations in 2005 suggested that cracks in Enceladus' southern hemisphere released plumes of ice and water vapor. A heat map (←) made from data collected in 2008 suggest that the cracks have temperatures as warm as 180 K (-93°C) compared to a general surface temperature averaging 75 K. Ultraviolet spectrograph data suggests that the plume is mostly gaseous water, with methane, carbon monoxide, carbon dioxide, and a mix of both simple and complex organic molecules.
It was first suggested that the heat is generated by friction when the fissures' walls rub against each other as Saturn exerts varying gravitational pull. But added data suggests rather that the heat is likely generated closer to the moon's core, perhaps by the flexing of the moon as it orbits Saturn. Saturn's E ring may be composed of material ejected from Enceladus by this plume process.
 This is almost trivial, but if the mechanism used by Enceladus to generate heat isn't immediately clear, recall that if one's hands get cold when out in winter weather, they can be warmed by briskly rubbing them together.
This is almost trivial, but if the mechanism used by Enceladus to generate heat isn't immediately clear, recall that if one's hands get cold when out in winter weather, they can be warmed by briskly rubbing them together.
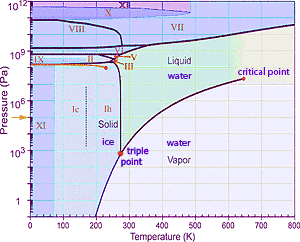
Its apropos to consider the formation of gaseous water vapor at the cold temperature of 180 K. Here on Earth we are familiar with water melting at 273 K (0°C) and boiling at 373 K. If you start at the gold arrow→ at right on the phase diagram at a pressure of one atmosphere (105Pa), moving to the right corresponds to warming solid ice with it melting to liquid water, then at 100° higher temperature boiling to water vapor. But at the much lower atmospheric pressure on Enceladus (lower on the diagram), water can sublimate directly from solid ice to water vapor.
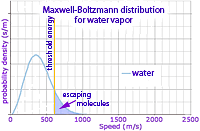 While even at 180 K the ice may be too cold to boil. However molecules are not all moving (vibrating) at the same speeds in the crystal lattice. The numbers of molecules with each speed are distributed (blue curve) as described by the statistical equation of Maxwell and Boltzmann. So while the water molecules with the most common speed (curve's peak) are not moving sufficiently fast to escape, a significant number of the fastest water molecule exceed the threshold energy necessary.
While even at 180 K the ice may be too cold to boil. However molecules are not all moving (vibrating) at the same speeds in the crystal lattice. The numbers of molecules with each speed are distributed (blue curve) as described by the statistical equation of Maxwell and Boltzmann. So while the water molecules with the most common speed (curve's peak) are not moving sufficiently fast to escape, a significant number of the fastest water molecule exceed the threshold energy necessary.
The same selection of the fastest molecules escaping occurs when we step out of a hot bath or shower and feel cooled. Only the fastest molecules escape and evaporate, so unless a heat source replaces that energy, those remaining have a lower average speed as measured by a cooler temperature.
Finally, it might be noted that while we are familiar with the form of solid ice common on Earth, the diagram indicates that under greater pressure, water uses different crystal lattice structures. But the ice on Enceladus is probably the same lattice as is familiar on Earth.
![]()
to planetary chemistry menu
to e-Chemistry menu
to site menu