![]()
![]()
Metals conduct electricity, a property essential to our high technology culture. Copper and to a lesser extend silver, gold, and other metals carry electric currents through virtually all our electronic appliances and machinery. But all such devices are manufactured as separate parts and connected together to function. One of the primary ways to electrically connect two metal parts is by adding a molten metal which hardens to a durable metal bridge when cooled. To provide such a function, a solder |from Latin, 'sä•d∂r| needs to have a melting point significantly below that of the other components, at a temperature which will not damage any of the nearby parts when it as applied as a molten liquid. Pure metals generally melt at too high a temperature to provide such a function. However when an impurity is added to a pure substance, generally the melting point of the combined material will be lower than that of the pure substance. Often the temperature needed to melt such a mixture is significantly less than the melting points of either of the component materials.
Tin is a metal which melts at 231°C and Lead melts at 327°C. When the two are combined, an alloy is formed with a lower melting point determined by the mixture's composition. Such solder is the linchpin of electronics manufacturing,
says Jack Geibig, acting director of the Center for Clean Products and Clean Technologies at the University of Tennessee. Without it, it's difficult to achieve a proper electronic connection that is durable and reliable.
Lead mixed with Tin has been ideal for solder. In fact, says Carol Handwerker, chief of the metallurgy division at the National Institute of Standards and Technology, The whole electronics infrastructure was designed around the melting point and physical properties of [Lead/Tin solder].
She notes that Lead is malleable and thus easy to work with, and it doesn't fracture. When Lead is combined with Tin in the ideal proportion (63% Tin, 37% Lead), the resulting alloy has a low melting point of 183°C, which is its great advantage. Geibig says: If you're not operating at really high temperatures, you have more control over processes, so that the processes aren't sensitive to slight temperature variations, which are costly to control.
Low temperatures also mean less strain on the equipment and materials such as printed circuit board and components that receive heat from the molten solder during the assembly process.
However Lead is one of the relatively rare (0.0013% of the earth's crust), heavy metals that biological systems have never adapted to either use or shed in anything above trace quantities. Humans first discovered they could obtain Lead from certain earthy materials about 6500 BC. The harmful effects of Lead were discovered as early as 2000 BC. But Lead was still used for fashioning vessels for eating and drinking, provide color in makeup, to sweeten wine, and provide a variety of medicinal uses. In recent times large quantities of Lead were used as colorants in paint and to maximize the proportion of distilled petroleum useful as fuel (gasoline for internal combustion engines). While Lead was known to poison the nervous system and also cause intestinal problems, more recently extreme learning disabilities were linked to relatively small consumption of Lead during early childhood. In living organisms the Lead combines with enzymes substituting for Calcium, Iron, and Zinc. However differences in Lead's electronegativity and size result in the enzymes either failing to function, or doing so at reduced rates. As a result of concern for Lead's toxicity, the use of Lead was abolished in both paints and fuels. But some countries have extended the ban of all uses of Lead and several other toxic metals.
EU Directive 2002/95/EC, the restriction of the use of certain hazardous substances in electrical and electronic equipment,
is also called RoHS, or simply, Pb-Free. While this initial legislation is from Europe, it is being adopted globally. The main impetus for the industry to avoid the use of Lead is the ban on Lead in electronics imposed by the European Union. Under the Restriction of Hazardous Substances directive, as of 1 July 2006 Lead must be replaced by other substances in electronic equipment. (The directive also bans Mercury, Cadmium, and the hexavalent Chromium ion.) After July 1, 2006, a product containing more than 0.1 percent Lead cannot be legally sold in the European Union. RoHS is primarily aimed at reducing the amount of Lead and five other chemicals commonly found in electronic components, cases and cabling from being dumped in landfills. Any electronic components bound for Europe are subject to the ban.
Lead is not a safety problem when contained in electronic equipment, says Robert Donkers, an environmental counselor for the European Commission who is based in Washington, DC. However, when electronic components are deposited in landfills, he speculates that people may scavenge for equipment, break it open, or the Lead may leach out of landfills and into drinking water. The risk is compounded in countries which import electronic waste. In China, for example, workers and children in a cottage recycling industry strip components out of discarded electronic devices. Lead exposure, even at low levels, is well known to harm children, as measured by lowered IQ. Lead also affects their ability to pay attention. Children exposed to low levels may also be hyperactive and irritable. As a result of these known effects, the United States has established a maximum allowable blood level for Lead of 10 micrograms per deciliter (μg/dL).
The effect of the Restriction of Hazardous Substances is the replacement of Lead/Tin solder with an alternate material which generally doesn't function as well. Its replacement is either pure Tin or other alloys.
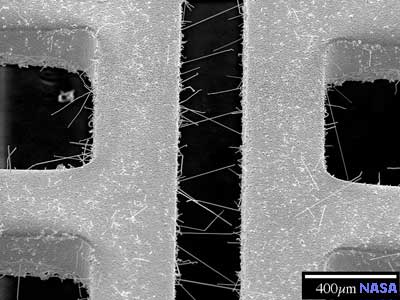
Where Tin plating is used, there is a risk of electrical failure. That is because pure Tin, Zinc and Cadmium all have the spontaneous property of developing filamentary crystal growths from the surface over time. These tiny filaments, referred to as whiskers,
can grow long enough to reach adjacent circuitry, thereby causing a short circuit so that the system fails to operate properly. The smaller whiskers vaporize, the vapor becomes energized as a plasma, and the plasma is conductive, able to carry HUNDREDS of amps of current, if only for a moment. That's believed to be enough to destroy most electronic devices. The smaller the whiskers the more dangerous they are. Even a straight Tin whisker short, no plasma, is reckoned to be about 50 milliamps, enough to bother a digital circuit. A problem like this could bring down a space station, a spy satellite or a large airplane like the giant Airbus A380 which is fly by wire. A significant number of communications satellite failures have already been attributed to Tin whiskers. As might be expected, NASA is very concerned about the use of pure Tin in products going into space since those products generally can't be easily repaired once they have been launched!
 |
I work for an electronics manufacturer, and Tin whiskers are just a nightmare. It is not that they arc and cause problems, they form a current bridge. 5 volts just isn't enough to vaporize them, but it is enough to make a part fail. I think we will be paying for this mistake for years to come.
Dr Henning Leidecker now at NASA Goddard (Greenbelt Md) (a former physics professor at American University speaking on the basis of his own experience,) wrote that:Our world depends on the proper functioning of electronics, and so this is a world-wide problem. We already see eliminations of Lead as causing failures in newly-produced electronics, and we can be sure we will see many more failures as the older equipment ages out of service and is replaced by this new Lead-free stuff.
Lives will surely be lost as a result, and probably already have been. But our systems are not set up to track this. Deaths are tracked at individual levels, but medical doctors assigning causes are not trained to report: Cause of death was the failure of electronics, which in turn failed because the Lead-free replacements did not work. Epidemic-tracking centers are set up to work with known diseases, and not deaths or injuries caused by equipment failing as a result of Lead-free substitutes.
Companies using the Lead-free replacements are not (as far as I know) reporting any injuries or deaths caused by the failures of their equipment, caused by Lead-free substitutions. I suppose they are more likely to settle any cases that are brought to their attention "out of court", which is to say, out of the public's attention. Consider the failure of Galaxy IV, that silenced 35 million communication devices for about a day. Some of these devices were used by medical doctors.
Can we suppose that there were NO cases of patient suffering (or worse) as a result of the loss of contact between patient and doctor? But who would track this? And make the findings publically available?
And companies that suffer as their products fail as a result of use of Leaded-Tin substitutes are not tracked either. While the company remains in business, it is typically reluctant to advertise that it is suffering from such problems. If the company dies, then so too does the reason. No one tracks this cause. No one can say, We lost 57 companies this year, as a result of failures due to the ill-performance of the Leaded-Tin substitutes.
So we have a world-wide situation that is under a basket, out of the light, and likely to remain that way!
One of the strong drivers for RoHS has been that people DO count the number of folks harmed by Lead in their environment. But we do not count the number harmed by removing Lead from electronics. So the situation is unbalanced.
Thus, one way to go forward in achieving a better balance in RoHS actions, is to add problems to people caused by failures of equipment caused by Lead-free substitutes to the problems noted by epidemic-tracking centers. Ditto for companies.
The problem with regulations, is that they are oft written by politicians; and obviously... their field of expertise differs.
Because of widespread litigation drawing attention to the issue, people will learn that even if {Lead-free} hardware were to become the only hardware available anywhere in the world, no cases of poisoning by Lead, or by any of the other prohibited substances, would be prevented because there never have been any cases from such use.
While the problems caused by Lead when ingested by people have been well documented, problems caused by the use of Lead in electronic products (including recycling) have not. Application of the precautionary principle to the substitute solder that the industry has adopted (referred to as SAC, a Lead-free alloy of Tin, Silver, and Copper), would require showing that its environmental and health impact is an improvement, or at least isn't worse. That hasn't happened in any systematic study.
I fix pianos. In the late 1950's, Steinway thought they could save money by using plastic parts in their piano actions. It worked very well until the late 1970's. Then there was a huge problem as all the plastic parts disintegrated, with some spectacular failures in concert. And pianos are easy and non-critical compared to debugging solder joints.
![]()
to Inorganic Chemistry menu
to e-Chemistry menu
to site menu