Physical Science
Evaluate Yourself and Improve
Chapter 3
Reminder: It is appropriate to occasionally think about what we hope to achieve and what we have learned already. This page is offered to assist with evaluating that progress.
Part I:
- Review the list (made at the end of Chapter 1) of what you hope this course helps you to understand.
- We use characteristic properties to identify parts of our world ranging from substances like water and copper to living creatures such as cats and human beings. Note that two things don't have to be totally identical to be identified as say trees, but they need to share characteristic properties. Consider which characteristics should be required to be human beings:
- Envision a universe with no characteristic properties. What would it be like if each substance and each object shifted properties perhaps at random?
- Start a list of useful characteristic properties.
Part II:
- What is the formula for calculating density?
- If a piece of metal has a mass of 16 grams and a volume of 2 cubic centimeters, then what would be its density?
- You can use density to identify different materials because
- the same kind of matter often has different densities.
- different kinds of matter have different densities.
- different kinds of matter always have the same density.
- different kinds of matter have different masses per weight.
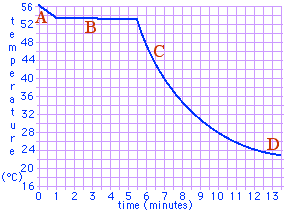
- White flakes of the compound para-dichlorobenzene are sometimes used as a moth repellant. If they are gently warmed they melt to a colorless liquid. At right is a graph of a liquid para-dichlorobenzene cooling in a bath of cold water. According to the graph, at what temperature does para-dichlorobenzene freeze?
- According to the graph, what is the temperature of the cold water bath?
- During which lettered line segment was the substance all liquid? ...all solid? ...partially liquid and partially solid?
- Determine which of the words describe characteristic properties of the substance; Then determine which of the words instead describe the properties of these particular objects rather than the substance of which they were made:
- The shiny, round, steel object...
- The warm, yellow, plastic ball...
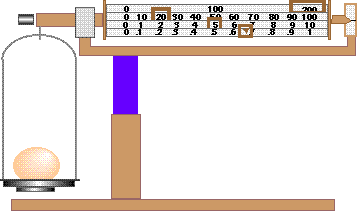
- What is the equipment shown here?
- What property does this equipment measure?
- What units does this equipment use for measuring?
- What is the mass of the egg?
- A 10.0 cm3 block of gold has a mass of 192 g. What is the density of this block?
- A sample of a liquid with a mass of 0.38 g occupies 0.50 cm3. What is its density?
- A student calculates the density of a metal cylinder as 7.8 g / cm3. What is the mass of this cylinder?
- Write each of the following numbers in scientific notation (as a number between 1 and 10 times the appropriate power of 10).
- 1000
- 6,940,000
- 0.000,000,000,003
- 0.005,92
- Change the following from scientific notation back to regular numbers that don't use exponents:
- 106
- 3.1 x 104
Table 1: Densities of some Solids, Liquids, and Gases
(In Grams Per Cubic Centimeter)
| substance |
density |
|
substance |
density |
| Osmium |
22.5 |
|
ice |
0.92 |
| Platinum |
21.4 |
|
Lithium (metal) |
0.53 |
| Gold |
19.3 |
|
liquid Helium (-269°C) |
0.15 |
| Mercury |
13.6 |
|
carbon dioxide |
1.8 x 10-3 |
| Lead |
11.3 |
|
Oxygen |
1.3 x 10-3 |
| Copper |
8.9 |
|
air |
1.2 x 10-3 |
| Iron |
7.9 |
|
Nitrogen |
1.2 x 10-3 |
| Aluminum |
2.7 |
|
Helium (as a gas) |
1.7 x 10-4 |
| water |
1.0 |
|
Hydrogen |
8.4 x 10-5 |
- Which substance listed in Table #1 has the smallest density, oxygen, helium, or hydrogen?
- What is the density of lead? (remember to include the labels!)
- Will lead float or sink in a puddle of mercury?
- The density of a substance is 8.2 g / cm3. Is this substance most likely a solid, a liquid, or a gas:
- Another substance has a density measured to be 0.0017 g / cm3. Which substance in Table #1 is this most likely to be?
- How close to the density listed in Table #1 was your measure of water's density?
- Why does ice float on liquid water?
- A beaker contains 130 mL of water. Calculate the mass of this water? (Hint: Use information from table #1 above.)
- What is the characteristic number of wheels per bicycle?
A beaker contained some candle wax is shown after being melted and again after it was allowed to cool.
- During the cooling, the MASS of the wax a) decreased. b. stayed the same. c. increased.
- During the cooling, the VOLUME of the wax a) decreased. b. stayed the same. c. increased.
- During the cooling, the DENSITY of the wax a) decreased. b. stayed the same. c. increased.
- Under what conditions should you wear goggles to protect your eyes?
- On a hike up the Greywolf River, you find a granite mountain. Propose a way how YOU could easily find the density of the mountain?
![]()
![]()


