Chemistry
Reactions & Reason
Sample A-15 test
[The intent is not to provide the CONTENT you need to know, but samples of the KINDS of SKILLS and DEPTH of UNDERSTANDING you should have. In short, don't try to memorize the ANSWERS, but learn HOW TO FIGURE OUT THE ANSWERS! The questions on the test you will take will likely have different answers.]Answers follow!
A lab team mixes two colorless liquids together in a test tube like you did in Experiment A-2. They record the following statements. You decide if each statement is an observation, or an interpretation:
The resulting mixture produces a bright blue precipitate.The lab team has mixed the two chemicals. Decide whether each of the following statement is adequate evidence suggesting that a chemical reaction occurred, or what some other kind of change:
The test tube feels cooler after the liquids were mixed.
Ammonia gas was released by the chemicals.
Wavy lines between the two liquids is evidence the liquids did not dissolve.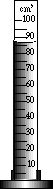 A yellow precipitate has formed.Determine the volume of liquid in the graduated cylinder accurately:
A yellow precipitate has formed.Determine the volume of liquid in the graduated cylinder accurately:
The mixture is noticably warmer than either original liquid.
The mixture has a noticable boundry between the top and bottom layers.
At first the mixture is clear, but over 10 minutes bubbles slowly form then rise to the surface.
The graph was plotted from data obtained from an experiment similar to A-7.
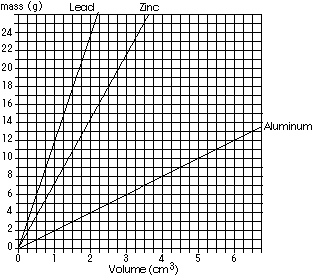 If 1.0mL of an unknown substance has a mass of 12g, what substance would it probably be? (Be careful of tricks; sometimes the real world IS tricky! You've got to be ready for the real world.)If 4cm3 of another unknown sample has a mass of 17g, what substance would it probably be?Based on the results of your experiment A-11, which of the following is a substance that sublimes? (Refer to you journal for assistance) SiO2, PbCl2, NaNO3, I2
If 1.0mL of an unknown substance has a mass of 12g, what substance would it probably be? (Be careful of tricks; sometimes the real world IS tricky! You've got to be ready for the real world.)If 4cm3 of another unknown sample has a mass of 17g, what substance would it probably be?Based on the results of your experiment A-11, which of the following is a substance that sublimes? (Refer to you journal for assistance) SiO2, PbCl2, NaNO3, I2
Which of the substances dissolves well in hexane but only poorly in water?In what way is the freezing point of a substance affected by impurities?
Your partner and you split responsibility for finding density of an unknown substance. Your partner reports that your empty graduated cylinder has a mass of 10.23 g. With 10.00 mL of liquid in the cylinder, the mass is 18.59 g. What is the liquid's density? [Your share of the work is to compute and correctly report the density, significant figures and all. SHOW WORK.]
A 120g mixture of NaNO3 and SiO2 contains 35g of NaNO3. Determine the percentage of silicon dioxide in the mixture. [As with all science problems, you need to show the logical steps the process starting with formulas, and substitutions of numbers into the formulas. While you need not show the actual calculations, that can be done on a calculator if you bring one, you must show your answer to the currect number of significant figures and labelled with the appropriate units.]
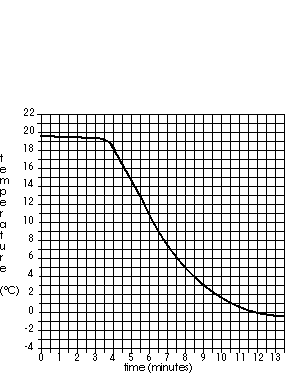
The graph to the right shows the temperature of a liquid being cooled in an ice bath similar to the procedure you used in A-13 and A-15. What is the temperature of the ice bath? What is this substances's freezing point?
Classify the following substances as mixtures or pure substance:
t-butanolConsider the following:
table sugar
orange juice
ice
tomatoesBased on this information, which chemical is most likely unknown Y?
substance density (g/cm3) freezing point (°C) boiling point (°C) acetic acid 1.049 16.6 118.1 t-butanol 0.789 25.5 82.8 cyclohexane 0.779 6.5 80.7 nitrobenzene 1.198 5.7 210.8 unkown Y 0.784 16.5 83.2 Lab safety and procedures: (Refer to the lab rules.)
If you obtain too much chemical, it is best to 1. return some to the original container. 2. throw some away. 3. use all of it in the emperiment.Weighing papers are 1. to keep balances clean. 2. to give directions how to use the balance. 3. to adjust balances to "zero."
Which of the following is important for safety? 1. Don't eat or drink in the lab. 2. Don't be a spectator of another lab team. 3. Don't sit on lab tables. 4. Wash hand after doing any experiment with hazardous chemicals. 5. Keep work areas clean.
Wear goggles 1. when heating substances warmer than boiling water. 2. around corrosive chemicals. 3. when using vacuum equipment. 4. when cutting or smoothing glass.
What is the SI unit for time?
Observations are made with the senses: can you see, smell, taste, hear or feel what is stated? Answers
Blue and precipitate (go to the bottom) are visible.Color changes, unexpected temperature changes, changes of state (solid, liquid,gas) not caused by temperature changes are all evidence of chemical change. But the boundary between layers is likely just an indication that the two chemicals did not dissolve and remain separate.
Cold if felt.
Ammonia has a distinctive odor; mention of its odor would have been a observation; but this does not describe any odor; it is probably an interpretation.
Wavey lines are visible.Read the bottom of the meniscus: 87°C
The first substance is likely lead. The second is probably something other than the three shown; remember there at many thousands of other chemicals in the world besides the three shown on this graph! You may recall from the precision of the class volume and mass measures, it was rare to be off from the the average by a whole mL or several grams. Precision is how tightly the points fit the average or the curve.
Iodine sublimes (when heated goes directly from solid to gas) and dissolves in hexane, but only slightly in water. Sand (SiO2) is insoluble in both, sodium nitrate dissolves in water. NOTE you ought to recognize (or have readily available in your journal) the chemical symbols and names for the substances you have used. Also, lead compounds were used extensively in the past, but because they can create health hazards if you eat or breath them (they are hard to get in your body any other way), their uses are being reduced.
If freezing points are affected at all (an impurity has an effect only if it dissolves) the freezing temperature is only LOWERED by impurities. You will learn later why this is.
18.59g - 10.32g = 8.27g (It is important to note that in this subtraction, we have lost some accuracy. The original measurements had 4 digits, but the difference only has 3 digits! Accuracy compares to the TRUE value; we often do not known the true value, but it is well known how various mathematical operations affect accurancy!) Density = mass/volume, so 8.37g/10.00mL = .837g/mL (Again note the accuracy of the answer. While the volume had 4 digits, the mass only had 3 digits. So the answer can be no better than the WORST of the measurements, only 3 digits. It is often preferable to write such a number as 0.837g/mL for the benefit for those with poor sight showing that there is a decimal point behind; the zero has no accuracy when it it left of all other digits: the answer still has 3 significant figures. Mathematics isn't perfect (yet); the zero can have different meanings; you will have to figure out when a zero indicates accuracy (also called significance) and when it is performing its decimal point duties.)
120g total - 35g sodium nitrate = 85g silicon dioxide. % = mass of interest/total mass so 85g/120g = 71% silicon dioxide. ALWAY include the formula in your work. It will help you keep track of what you are doing. In this case you are interested in silicon dioxide! Note the answer is rounded off to 2 significant figures.
Given enough time, the substance will cool to the freezer temperature, then remain (level) at that temperature. So the freezer is about -0.4°C or -0.5°C. When a pure substance freezed it remains at its frrzing temperature for a while (plateau on the graph). The less pure, the less level the plateau will be. The only plateau (other than the freezer temperature) is between 19°C and 20°C.
Mixtures have two or more materials which are often visible. Tomatoes have skin, pulp, seeds, etc, and orange juice often has pulp that floats to the top. You may also have made orange juice from concentrate in which you mix the orange frozen concentrate with colorless water. You cooled T-Butanol in an experiment and found it had a freezing plateau, a good sign of purity. Ice is pure water; in fact impurities can often be removed from water by freezing the water and rinsing off the ice. You may have learned in biology that table sugar is the pure substance sucrose. But you also know that sugar, T-butanol, and ice all look like single substances with no detectable second materials present.
T-butanol and cyclohexane have densities and boiling points close to that of substance Y. They are possible matches. The freezing points are not as close. But recall that impurities lower a freezing point, but NEVER raise it. In the experiment A-13 some classmates found that impurities may lower a freezing point a lot. Substance Y could be impure T-butanol with its freezing point lowered about 9°C, but could not be cyclohexane with a RAISED freezing point. Incidentally, boiling points ARE RAINSED by impurities, but typically not very much.
Discard excess reagents.
Keep the balances from getting corroded (an inaccurate) by using weighing paper.
All are important for safety.
Wear goggles for all these activities.Le Système International d'Unités (SI) has established basic, derived, and other (tolerated) units for measurement. The primary unit for time is the second.
Cheers, and good luck on the test!
.
(return to Chemistry Calendar)
.
7/5/99