



Gustav Robert Kirchhoff (born 1824 in Königsberg, Prussia, died 1887; ←shown at left) and Robert Wilhelm Bunsen (b1811 in Gottingen, d1899; shown at right→) invented a spectroscope to systematically study the effects of elements on flame color. In 1859, they found each element has spectral lines not effected by the presence of other elements
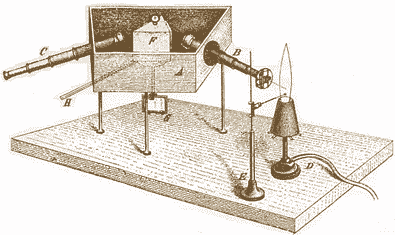
Their spectroscope (shown below ↓) combined a prism with a telescope. Different colors of light are separated so their colors and intensities can be studied. A tiny sample of the substance is held (by E) in a nearly colorless gas flame (from D), brightly coloring the flame with the spectral lines of the element(s) in the substance. A narrow beam of light from the flame is concentrated by lenses (in B), focused on the prism (F) which is moveable (by handle H) so each spectral line can be viewed distinctly (through magnifying lenses in C). The enclosed box (A) and focusing tubes (B and C) keep out stray light.
 Spectral analysis proved to be a very productive tool for discovering elements present in only trace amounts. Robert Bunsen found two new elements, one he named Cesium for its blue spectral line, and the second named Rubidium for its red spectral line. Caesius is Latin for sky blue. Rubidius is Latin for deepest red.
Spectral analysis proved to be a very productive tool for discovering elements present in only trace amounts. Robert Bunsen found two new elements, one he named Cesium for its blue spectral line, and the second named Rubidium for its red spectral line. Caesius is Latin for sky blue. Rubidius is Latin for deepest red.
 Sir William Crookes (b1832 in London, d1919; shown at right→) discovered the element which he named Thallium describing its primary spectra color. Thallos is Greek for green shoot or twig.
Sir William Crookes (b1832 in London, d1919; shown at right→) discovered the element which he named Thallium describing its primary spectra color. Thallos is Greek for green shoot or twig.
In 1863 Ferdinand Reich (b1799 in Bernberg Germany, d1882, ←portait at left) and Hieronymous Theodor Richter (b1824 in Dresden, d1898; who identified the colors for his older color blind partner) discovered an element with an indigo spectral line which they named Indium. Indigo is a violet-blue dye made from pea plants.
The advent of the spectroscope accelerated the discovery of both very reactive and thus difficult to separate elements as well as the non-reactive rare earths. Spectral analysis requires just a small amount of material, which if often the case when a new element is first produced. It also can be used for analysis of substances glowing at great distances from the observer. This feature resulted in the element Helium being discovered on the Sun before it was isolated on Earth. It also allows astronomers to chemically analyze the most distance objects in the universe.


| introduction | Greeks | alchemy | Lavoisier | Dalton | Berzelius | molecules | ↑ | electron | radiation | Bohr | isotopes | synthesis |
| to site menu | Discovery and Naming of Chemical Elements |
chemistry | physics | |||||||||
| created 23 March 2002; additions 24 March latest revision 27 April 2010 |
by D Trapp | |||||||||||