
Screens coated with fluorescent material provided a convenient way to make visible the path of cathode rays (eventually found to be a stream of particles now called electrons) generated inside a well evacuated Crookes' tubes by high voltage electric discharges.

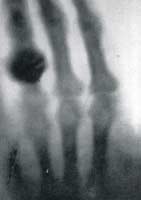 November 8, 1895 Wilhelm Konrad Röntgen (born 1845, died 1923; photograph at right→) noted that while he was generating cathode rays inside a Crookes' tube, nearby (up to 2 m away) paper coated with barium platinocyanide lit up with brilliant fluorescence. Knowing that the cathode rays could not penetrate the glass tube, Röntgen realized some unknown radiation, which he called X-rays, must pass through glass and air to excite the nearby fluorescence crystals. The rays originated from within the glowing Crookes' tube. Röntgen stayed up all night experimenting and for a time ate and slept in the laboratory. Röntgen found X-rays exposed photographic film. He found X-rays pass through low density materials such as the soft tissue of a hand but less through materials containing elements with higher atomic weight, so that bones would cast a shadow on fluorescent materials or photographic emulsions. (←at left Röntgen's first X-ray photograph, click to enlarge) Within the next few days he demonstrated that the X-rays have some of the characteristics of high energy electromagnetic waves. (Electromagnetic radiation ranges from high energy, short wave-length gamma and X-rays, through ultraviolet light, visible light, and infrared, to low energy, long wave-length radio waves.) He considered whether X-rays could be a longitudinal wave form of electromagnetic radiation. He concluded that if X-rays are a kind of light, then they differed from the then highest energy known form of electromagnetic wave, ultraviolet light, in the following ways.
November 8, 1895 Wilhelm Konrad Röntgen (born 1845, died 1923; photograph at right→) noted that while he was generating cathode rays inside a Crookes' tube, nearby (up to 2 m away) paper coated with barium platinocyanide lit up with brilliant fluorescence. Knowing that the cathode rays could not penetrate the glass tube, Röntgen realized some unknown radiation, which he called X-rays, must pass through glass and air to excite the nearby fluorescence crystals. The rays originated from within the glowing Crookes' tube. Röntgen stayed up all night experimenting and for a time ate and slept in the laboratory. Röntgen found X-rays exposed photographic film. He found X-rays pass through low density materials such as the soft tissue of a hand but less through materials containing elements with higher atomic weight, so that bones would cast a shadow on fluorescent materials or photographic emulsions. (←at left Röntgen's first X-ray photograph, click to enlarge) Within the next few days he demonstrated that the X-rays have some of the characteristics of high energy electromagnetic waves. (Electromagnetic radiation ranges from high energy, short wave-length gamma and X-rays, through ultraviolet light, visible light, and infrared, to low energy, long wave-length radio waves.) He considered whether X-rays could be a longitudinal wave form of electromagnetic radiation. He concluded that if X-rays are a kind of light, then they differed from the then highest energy known form of electromagnetic wave, ultraviolet light, in the following ways.
His preliminary communication
was presented before the end of the year. (A translation of the original German publication is among Carmen Guinta's classic papers.) On New Year's Day 1896, Röntgen mailed copies of his paper and photographs to a number of other scientists.
 At the weekly meeting of the French Academy of Science in Paris on the evening of January 20, 1896, Henri Poincaré describe the recent discovery of X-rays and show photographs of living bone that Röntgen had taken. Poincaré wondered out loud during his talk if X-rays were emitted by other luminescent bodies. Antoine Henri Becquerel (b1852, d1908; at right→) undertook to investigate that hunch the next day. His father had been an authority on phosphorescence for many years. Henri himself had studied phosphorescence including that of some Uranium compounds and had photography experience. It was proposed that a phosphorescent mineral would augment the cathode rays or ultraviolet light creating the X-rays. Becquerel and others presumed a substance had to luminesce in order to emit X-rays.
At the weekly meeting of the French Academy of Science in Paris on the evening of January 20, 1896, Henri Poincaré describe the recent discovery of X-rays and show photographs of living bone that Röntgen had taken. Poincaré wondered out loud during his talk if X-rays were emitted by other luminescent bodies. Antoine Henri Becquerel (b1852, d1908; at right→) undertook to investigate that hunch the next day. His father had been an authority on phosphorescence for many years. Henri himself had studied phosphorescence including that of some Uranium compounds and had photography experience. It was proposed that a phosphorescent mineral would augment the cathode rays or ultraviolet light creating the X-rays. Becquerel and others presumed a substance had to luminesce in order to emit X-rays.
Becquerel studied several different phosphorescent substances, but with no results. However he was hopeful for success with Uranium compounds, which emit and absorb a whole series of harmonic luminous radiations, seem to have a particularly remarkable molecular constitution, at least from the point of view of absorption and phosphorescence.
Having loaned out his samples of Uranium, he did not begin investigating phosphorescent Uranium compounds for several weeks. At the February 24, 1896 meeting of the French Academy of Science, he claimed success, reporting that uranium potassium sulfate (after being made to luminesce by sunlight) emitted rays which reduced the silver bromide emulsion on a photographic plate. He had placed coins and other objects under the crystals and the shapes were reproduced on the photographic plate.
Becquerel's technique was to double wrap a photographic plate in black paper or cloth to keep out all but X-rays, tape Uranium salts on top and lay the apparatus on the window sill where ultraviolet from the sun would cause the salt to fluoresce. After a prolonged exposure to ultraviolet light, he would develop the photographic plate and inspect for exposure caused by the penetrating X-rays. On February 26 and 27 Becquerel again prepared plates coated with silver bromide, wrapped in black cloth, with uranium potassium sulfate [SO4(UO)K+H2O] salts taped on top. But when it began to rain and threaten the apparatus, Becquerel suspended the experiment, placing the salt with the wrapped photographic plate underneath in the darkness of a bureau drawer to await the return of the sun. Paris remained wet and overcast several days. Becquerel developed the photographic plate on Sunday, probably hoping to find a slight exposure caused by the brief fluorescence which he might report to the French Academy of Science on Monday. Becquerel reported: I developed the photographic plates on the 1st of March, expecting to find the images very weak. Instead the silhouettes appeared with great intensity. I immediately thought that the action had to continue in darkness...
...these rays, whose effects have a great similarity to the effects produced by the rays studied by Röntgen, are invisible rays emitted by phosphorescence and persisting infinitely longer than the duration of the luminous rays emitted by these bodies. However, the present experiments, without being contrary to this hypothesis, do not warrant this conclusion.
i.e., Becquerel realized that his experiments found rays SIMILAR to X-rays, but the experiments did not establish that these rays generated without any initializing energy ARE X-rays. (Carmen Guinta translates the original French papers.) The term radiation which Becquerel meant to be generic became the specific term that is still applied to these rays.
French physicist Pierre Curie (b1859, d1906) had developed a very sensitive electrometer that could be used to measure this new radiation by timing how rapidly an electric charge leaked away. Pierre's wife, Marie Sklodowska Curie (b1867, d1934), did a survey of other substances and found that thorium also emits radiation. (Carmen Guinta translates their paper.) Finding the mineral pitchblende (primarily uranium potassium sulfate) to be more radioactive than pure uranium, it appeared it might contain additional radioactive substances. Marie, trained as a chemist but unemployed, chemically separated the pitchblende to isolate the cause of the more intense radioactivity. Marie isolated two new elements: The first they named Polonium after Marie's native country, Poland, then a nonexistent country having been absorbed by Russia! The second was named Radium because of its intense radioactivity. (The Classic Chemistry Papers by Carmen Guinta contains their papers announcing Polonium and Radium. Photo ar right was taken while they were on their honeymoon via bicycle.)
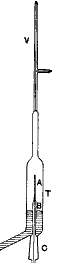
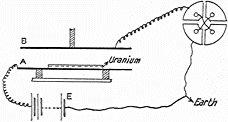 Formerly a New Zealand student at Trinity College, Cambridge, having left England for a job as Professor of Physics, McGill University, Montreal, Ernest Rutherford (b1871, d1937) noted that the radiation from uranium which caused electric charge to leak away could be blocked by covering with increasing thicknesses of aluminum. (apparatus at right) But after much of the radiation was blocked, additional aluminum reduced the remaining radiation much more gradually. In 1899 he concluded that uranium must emit two distinct types with differing penetrating powers. Rutherford named these using the Greek alphabet α (pronounced
Formerly a New Zealand student at Trinity College, Cambridge, having left England for a job as Professor of Physics, McGill University, Montreal, Ernest Rutherford (b1871, d1937) noted that the radiation from uranium which caused electric charge to leak away could be blocked by covering with increasing thicknesses of aluminum. (apparatus at right) But after much of the radiation was blocked, additional aluminum reduced the remaining radiation much more gradually. In 1899 he concluded that uranium must emit two distinct types with differing penetrating powers. Rutherford named these using the Greek alphabet α (pronounced alpha
), and β (pronounced beta
). (See E. Rutherford, Uranium Radiation and the Electrical Conduction, in the Philosophical Magazine, January 1899) β radiation has the characteristics of fast electrons. In 1900 the French physicist Paul Villard (1860-1934) found an even more penetrating type of radiation which was called γ (pronounced gamma
). He recognized γ has the properties of X-rays.
Ernest Rutherford and Frederick Soddy (b1877, d1956) suggested in 1902 that emission of radiation from radioactive materials results in the formation of new elements. (See E. Rutherford & F Soddy, The Cause and Nature of Radioactivity, in Philosophical Magazine)
In 1909 Rutherford and Thomas Royds (b1884, d1955) captured α radiation from radium, compressed the resulting gas above mercury flooded into the apparatus (sketched at left), and identified its spectra excited by a spark to be that of Helium. (See E. Rutherford & T. Royds, The Nature of the a Particle from Radioactive Substances, Philosophical Magazine)


| introduction | Greeks | alchemy | Lavoisier | Dalton | Berzelius | molecules | spectra | electron | ↑ | Bohr | isotopes | synthesis |
| to site menu | Discovery and Naming of Chemical Elements |
chemistry | physics | |||||||||
| created 23 March 2002 latest revision 1 May 2010 |
by D Trapp | |||||||||||