
Between 1855 and 1860 Heinrich Geissler (born 1814, died 1859) developed a Mercury diffusion pump producing high vacuums. He produced electric discharge between electrodes sealed in evacuated glass tubes. Julius Plücker (b1801, d1868) noted fluorescence on the glass when an electric spark passed through Geissler tubes. The fluorescence could be moved with a magnet. This was consistent with the idea that moving bodies carrying electric charge should be deflected in a magnetic field. But such charged bodies should also be deflected by metal plates charged with electricity. But attempts to demonstrate such electrical deflection had all failed.
J.W. Hittorf noted that the electric discharge in Geissler tubes produces shadows behind obstacles. The location of the shadows suggested that the sparks originated from the negative electrode and moved towards the positive electrode.
 In 1874 William Crookes turned a small paddle wheel with the discharge. He believed the rays were negatively charged material, perhaps a fourth state of matter. (As a result they were called Crookes rays by many scientists.)
In 1874 William Crookes turned a small paddle wheel with the discharge. He believed the rays were negatively charged material, perhaps a fourth state of matter. (As a result they were called Crookes rays by many scientists.)
In 1883 Svante Arrhenius (b1859, d1927, ←photograph at left) presented to the Swedish Academy of Sciences his theory that neutrally charged salts dissociate when dissolving to form electrically charged atoms or groups of atoms called ions. The ions explained electrical conduction of solutions of salts, anomalies in osmosis, and similarities in the heats of neutralization. Arrhenius's theory eliminated much of the confusion about the properties of solutions and indefinite compounds which had discouraged Mendeléeff and others from fully accepting the atomic theory. Still the atomic theory continued to remain controversial for many chemists and physicists until well after 1900.
After Arrhenius introduced his theory about charged ions, many suspected that the current in Geissler's tubes is likely carried by charged atoms moving in the near vacuum similar to the current carried in solutions.
Eugen Goldstein (b1850, d1930) proposed the negative (–) radiation be called cathode rays. Behind holes bored in the cathode he found opposite charged (+) radiation in 1886 for which he proposed the name canal rays. In 1891 George Johnstone Stoney (b1826, d1911) proposed the unit of charge should be called an electron.
 In 1897 Wilhelm Wien (b1864, d1928) measured the mass of the positively charged canal rays, finding they have the mass of ions. Thus gases (at least at very low pressures), like solutions, do use ions to conduct electricity.
In 1897 Wilhelm Wien (b1864, d1928) measured the mass of the positively charged canal rays, finding they have the mass of ions. Thus gases (at least at very low pressures), like solutions, do use ions to conduct electricity.
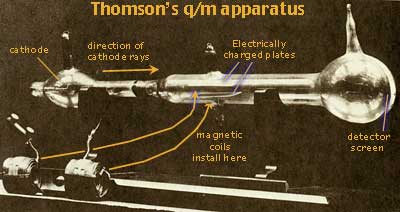
In 1897 Joseph John Thomson (b1856, d1940, ←photograph at left) at Cambridge, suspected that residual air stuck to the internal tube parts might shield the rays from electric fields thus blocking the expected attraction or repulsion by charged plates. Thomson baked the tubes to release residual gases prior to final evacuation. Having done so, he was finally able to deflect the cathode rays by an electric field. Adjusting the strength of an electric field created by charged metal plates inside his apparatus (shown below ↓) to just counteract a magnetic field created by two magnetic coils of wire placed outside straddling the apparatus, Thomson was able to cancel the effects of the two forces so that a beam of cathode rays travelled straight through his apparatus. Then combining equations for electric and magnetic forces, he calculated the speed of the negatively charged cathode rays, and the ratio of their electric charge to mass (q/m). The speed was near that of light. The q/m ratio was consistent with a mass less than 1/1000 that of the lightest atom, Hydrogen. Thomson proposed electric corpuscle as the name of particles constituting cathode rays, but Goldstein's name electron became popular instead. J.J. Thomson subsequently found that identical electrons were obtained from every kind of metal electrode he tried. Thomson concluded that electrons are probably a fundamental part of all atoms.
J.J. Thomson and William Thomson (Lord Kelvin) proposed a model for atoms suggesting that atoms are built like plum pudding. Negative electrons are like raisins evenly distributed through positively charged pudding. (Plum pudding is a common English steamed bread pudding well mixed with various nuts and fruits)
Electron,or Atom of Electricity, Philosophical Magazine, 1894, posted on ChemTeam web site.


| introduction | Greeks | alchemy | Lavoisier | Dalton | Berzelius | molecules | spectra | ↑ | radiation | Bohr | isotopes | synthesis |
| to site menu | Discovery and Naming of Chemical Elements |
chemistry | physics | |||||||||
| created 23 March 2002 latest revision 1 May 2010 |
by D Trapp | |||||||||||