![]()
![]()
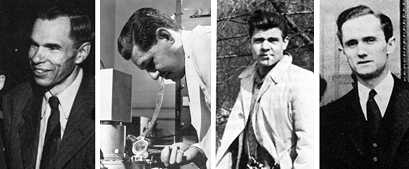 Elements #95 (Americium, Am) and #96 (Curium, Cm) were made and identified in 1944 by Glenn T. Seaborg, Albert Ghiorso, and Ralph A. James (shown here from left with Tom Morgan on right). As part of the secret Manhattan project to make an atomic bomb to end World War II, Seaborg headed a group of chemists at the Metallurgical Laboratory in Chicago. Their task was to work out chemical processes to extract Plutonium (#94) produced when Uranium (#92) had been bombarded with neutrons. In December 1943, Seaborg thought that satisfactory progress on Plutonium extraction allowed an attempt to synthesize heavier elements.
Elements #95 (Americium, Am) and #96 (Curium, Cm) were made and identified in 1944 by Glenn T. Seaborg, Albert Ghiorso, and Ralph A. James (shown here from left with Tom Morgan on right). As part of the secret Manhattan project to make an atomic bomb to end World War II, Seaborg headed a group of chemists at the Metallurgical Laboratory in Chicago. Their task was to work out chemical processes to extract Plutonium (#94) produced when Uranium (#92) had been bombarded with neutrons. In December 1943, Seaborg thought that satisfactory progress on Plutonium extraction allowed an attempt to synthesize heavier elements.
In January 1944 Seaborg and James bombarded 0.1 mg and 1 mg samples of Plutonium with deuterons using the Washington University cyclotron. Subsequent sample measurements searching for the expected higher energy alpha radiation than Plutonium´s detected none. Suspecting that chemical separation would be necessary for detection, additional samples were oxidized and separated. Elements #92, #93, and #94 formed insoluble fluorides in the III and IV oxidation state, but the VI state had soluble fluorides. But no unique alpha radiation energies were detected. (It is now understood that element #95 and #96 isotopes produced by the deuterons are primarily beta emitters, so the search was for the wrong evidence.)
At the same time, small samples of Pu-239 were further irradiated with neutrons in the small test nuclear reactor at Clinton, Tennessee, to see if Pu-240 might form, then decay by beta emission to form element #95-240. Ghiorso began to divert some of his time to help with detection. (Pu-240 remained undetected because it´s alpha radiation is too similar to that from Pu-239.)
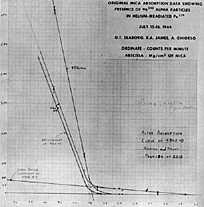 By July, Seaborg began to realize that rather than treating elements #95 and #96 as similar to elements #92, #93, and #94, they might be a series parallel to the Lanthanides. Elements #95 and #96 might be chemically similar to Europium (#63) and Gadolinium (#64) and not oxidize to the soluble VI state. (See Seaborg's revised periodic chart.) In early July the Berkeley 60" cyclotron was able to bombarded Pu-239 with 32 Mev Helium ions. The sample was flown to Chicago where 2.2 mg of Pu-239 was repeated oxidized to VI and removed three times. Added lanthanum fluoride carried any trace insoluble product. The insoluble product still contained about 0.09 mg of Pu causing about 12,000 alpha emissions per minute. On July 14, 15, and 16, graphing the number of alpha verses multiple layers of mica sheets revealed a more penetrating (higher energy) alpha emission than that characteristic of Pu-239. (Note the small amount of radiation on the graph that trails out to the right penetrating more mica than the more intense, but rapidly stopped Pu-239 radiation.)
By July, Seaborg began to realize that rather than treating elements #95 and #96 as similar to elements #92, #93, and #94, they might be a series parallel to the Lanthanides. Elements #95 and #96 might be chemically similar to Europium (#63) and Gadolinium (#64) and not oxidize to the soluble VI state. (See Seaborg's revised periodic chart.) In early July the Berkeley 60" cyclotron was able to bombarded Pu-239 with 32 Mev Helium ions. The sample was flown to Chicago where 2.2 mg of Pu-239 was repeated oxidized to VI and removed three times. Added lanthanum fluoride carried any trace insoluble product. The insoluble product still contained about 0.09 mg of Pu causing about 12,000 alpha emissions per minute. On July 14, 15, and 16, graphing the number of alpha verses multiple layers of mica sheets revealed a more penetrating (higher energy) alpha emission than that characteristic of Pu-239. (Note the small amount of radiation on the graph that trails out to the right penetrating more mica than the more intense, but rapidly stopped Pu-239 radiation.)
The element 96 (now called Curium, Cm) had been produced by the reaction:
But in July, the new element(s) had been detected by not yet identified. Likely the 24396Cm immediately decayed by either emission of a proton or neutron making either 24295Am or 24296Cm. As the war effort ramped up Plutonium production for building and testing an atomic bomb, larger samples produced by neutron absorption became available for testing. 8.2 mg came from the Clinton reactor in November and a 25 mg sample exposed to high neutron flux arrived from Hanford in January 1945. These larger samples suggested that there were TWO distinct high energy alpha emissions.
 By March 24194Pu was produced, separated, and weighed. It was found to decay by beta emission to 24195Am which emitted alpha emission equalling the lesser of the two long ranges. Additional isotopes of the element suspected to be #96 were produced by an upgraded Berkeley 60" cyclotron. By July one of the alpha decay products was identified as 23894Pu further confirming the existence of 24296Cm.
By March 24194Pu was produced, separated, and weighed. It was found to decay by beta emission to 24195Am which emitted alpha emission equalling the lesser of the two long ranges. Additional isotopes of the element suspected to be #96 were produced by an upgraded Berkeley 60" cyclotron. By July one of the alpha decay products was identified as 23894Pu further confirming the existence of 24296Cm.
Seaborg arranged to declassify the discovery of elements #95 and #96 for announcement at a November American Chemical Society meeting. But he appeared as a guest on the radio show Quiz Kids
the Sunday before the meeting. In the informal time after the quiz, one of the students asked: Have there been any other new elements discovered like Plutonium and Neptunium?
To which Seaborg responded on live radio: Oh yes, Dick. Recently there have been two new elements discovered—elements with atomic numbers 95 and 96—out at the Metallurgical Laboratory here in Chicago.
Tom Morgan, who joined the team in September 1944 referred to elements #95 and #96 as Pandemonium
and Delirium.
But in a talk to the April 1946 ACS meeting, Seaborg gave the name Americium (symbol Am) after the Americas, in analogy to the naming of its rare-earth homologue Europium after Europe. For element #96 we suggested the name Curium (symbol Cm) after Pierre and Marie Curie, in analogy to the naming of its homologue Gadolinium after Johan Gadolin.
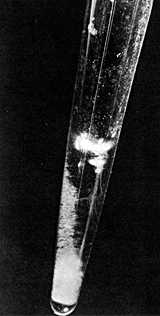
The first visible amount (30 micrograms shown in photo) was isolated by Werner and Perlman of the University of California in 1947. Energy from radioactive decay excites electrons causing Curium to glow in the dark!
![]()
![]()
| introduction | alchemy | planets | other celestial objects | color | other properties | myths | minerals | ore mines | other places | combination names | people |
| to site menu | Introduction to Development of Periodic Chart |
18th Century vocabulary, & index of people |
chemistry | physics | |||||||
| created 31 December 2000 content revised 30 December 2001 links revised 14 June 2007 |
by D Trapp | ||||||||||