![]()
![]()

Lightning preceded life on earth. Benjamin Franklin (b1706, d1790 portrait at right→) and others suggested that lightning was a giant electrical spark. Franklin had proposed that there is only a single kind of electricity which in excess or deficiency accounted for the two opposing electric effects observed by others. Franklin suggested a spark is the flow of electricity from locations of excess to deficient locations. Carefully observing sparks in open air, Franklin attempted to correctly label what he called positive and negative. He figured that the spark must travel from the positive (excess of charge) to negative (deficiency of charge) so the challenge was to observe the direction a very fast spark moves. Franklin admitted that the speed was so fast that the direction of motion was nearly a guess. But Franklin made the guess and got the names we still use, positive and negative, exactly backwards.
In 1800 Alesandro Volta (b1745, d1827) found a way to produce a steady flow of electrical current. That made it possible for André-Marie Ampère (b1775, d1836), Hans Christian Oersted (b1777, d1851), Michael Faraday (b1791, d1867) and Carl Friedrich Gauss (b1777, d1855) to determined the laws of electricity and magnetism which were restated and completed by James Clerk Maxwell (b1831, d1879, below left ↵) by 1862. Michael Faraday had found a way to produce electricity more cheaply.
 Yet in the second half of the 19th Century, it was clear that the fundamental nature of electricity was still poorly understood. Just as the earlier progress was the consequence of Volta's discovery of a way to produce a steady current, further progress followed the development of another new technology, in this case von Geurek's development of an efficient vacuum pump. Producing a high vacuum allowed Sir William Crooks (b1832, d1919) to construct evacuated glass tubes and send long electric sparks through the very low pressure gases contained therein. The cathode rays would be accelerated by an electric field: F = qE where q is the electric charge and E the electric field strength. This apparatus allowed experimenters to investigate the nature of electric current by visually observing the effects of barriers, pin wheels, magnetic fields, an such possible influences. For example, a barrier casts a shadow on the back side. It is clear from which side has the shadow that the spark travels from the negative electrode towards the positive electrode. So contrary to Franklin's guess, the electric charge carried by a spark is negative. Using Faraday's vocabulary, the spark was emitted from the cathode, so the charge carriers were dubbed cathode rays. But a spark in a Crooks tube will also turn a light pin wheel demonstrating that these cathode rays carry momentum and so probably have mass.
Yet in the second half of the 19th Century, it was clear that the fundamental nature of electricity was still poorly understood. Just as the earlier progress was the consequence of Volta's discovery of a way to produce a steady current, further progress followed the development of another new technology, in this case von Geurek's development of an efficient vacuum pump. Producing a high vacuum allowed Sir William Crooks (b1832, d1919) to construct evacuated glass tubes and send long electric sparks through the very low pressure gases contained therein. The cathode rays would be accelerated by an electric field: F = qE where q is the electric charge and E the electric field strength. This apparatus allowed experimenters to investigate the nature of electric current by visually observing the effects of barriers, pin wheels, magnetic fields, an such possible influences. For example, a barrier casts a shadow on the back side. It is clear from which side has the shadow that the spark travels from the negative electrode towards the positive electrode. So contrary to Franklin's guess, the electric charge carried by a spark is negative. Using Faraday's vocabulary, the spark was emitted from the cathode, so the charge carriers were dubbed cathode rays. But a spark in a Crooks tube will also turn a light pin wheel demonstrating that these cathode rays carry momentum and so probably have mass.
In 1897 Sir Joseph John Thomson (b1846, d1940, photo below left ↵), head of the famous Cavendish Laboratory at Cambridge University, devised a series of experiments which demonstrated that the spark is a beam of identical particles and determined the mass of those particles. If the cathode rays travelled between a pair of parallel metal plates carrying opposite charges, they should be deflected, attracted to the positive and repelled by the negative charges on the plates. But others had had difficulty demonstrating this effect. Thomson found that if he baked the apparatus after it have been evaluated to a vacuum, he could remove surface impurities from the plates and demonstrate the effect of the electric field on the spark. The electric field provided a force of acceleration at right angles to the motion of the cathode rays, a centripetal force: F = mv2/R where m is the particle's mass and R the radius of path curvature. The basic concept is that a particle's mass resists being deflected into a curved path. So measuring the curve gives a measure of the mass of the cathode rays.
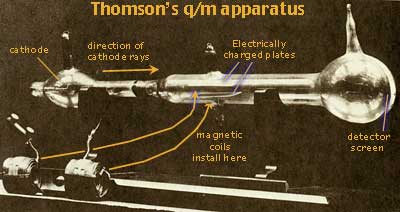 Thompson's beam of cathode rays always ended at only a single spot on a screen at the end of the long evacuated tube. So the spark is only composed of identical particles. And all negatively charged metal cathodes emitted identical, charge carrying particles!
Thompson's beam of cathode rays always ended at only a single spot on a screen at the end of the long evacuated tube. So the spark is only composed of identical particles. And all negatively charged metal cathodes emitted identical, charge carrying particles!
 A spark is also effected by a magnetic field: F = q B x v. This is a cross product where the force is at right angles to both the magnetic field, B, and the beam velocity, v. By arranging for the electric field to exert a force one direction (say upward) and a magnetic field to exert a counterbalancing force (say downward), Thomson was able to adjust the two forces so the beam of cathode rays continued a straight unaffected path. By balancing the known electric and magnetic fields so their effects cancelled, J.J.Thomson was able to set the equations equal and to determine the charge to mass ratio of the cathode rays: q/m = 1.76 x 1011 coul/kg.
A spark is also effected by a magnetic field: F = q B x v. This is a cross product where the force is at right angles to both the magnetic field, B, and the beam velocity, v. By arranging for the electric field to exert a force one direction (say upward) and a magnetic field to exert a counterbalancing force (say downward), Thomson was able to adjust the two forces so the beam of cathode rays continued a straight unaffected path. By balancing the known electric and magnetic fields so their effects cancelled, J.J.Thomson was able to set the equations equal and to determine the charge to mass ratio of the cathode rays: q/m = 1.76 x 1011 coul/kg.
Today one might be tempted to substitute the estimated electric charge on a hydrogen atom into the ratio. But recall that some noted physicists would still vehemently doubted the reality of atoms for another decade. And in any case, the assumption that the chemical and physical charges are equivalent was considered rather weak. Still a hydrogen ion in an acidic solution has a charge to mass ratio q/m = 9.6 x 107 coul/kg. These numerical measurements at least hinted that either the charge of cathode rays is 1800 times larger than that on a hydrogen atom, or that the cathode ray had a mass only 1/1800 of that of the smallest atom. Thomson thought the latter. It was a revolutionary idea which implied that INSIDE the atom, previously defined as the smallest possible piece of matter, was a still smaller particle! J.J. Thomson suggested to name this piece of an atom the electric corpuscle. But the name that stuck was the electron.

 Between 1909 and 1916 the American Robert A. Millikan (b1868, d1953, ←at left) devised increasingly precise ways to demonstrate that there is a smallest possible electric charge and to measure that charge. Earlier Charles Wilson in England had devised what became known as a cloud chamber to visualize the path of charged particles by a trail of droplets. After an observation, the trail of charged droplets could be cleared from the apparatus by applying an electric field arranged by parallel plates similar to what Thomson use. But in air, the tiny droplets quickly reach a terminal velocity, and then travel at constant velocity. The accelerating force on their electric charge quickly is balanced by friction determined by the droplet size. Millikan realized that the experiment could be turned around so by measuring the size and terminal speed, he could calculate the electric charge on each droplet. But condensation or evaporation often changed the tiny droplet size complicating the calculations. While on a train journey to a convention, Millikan glanced at his watch and realized that the clock oil used for long lasting lubrication was chosen for its lack of evaporation! Substituting a fine mist of oil droplets (inside the pot above right→ to assure uniform temperature, block air currents and extraneous electric fields), Millikan demonstrated that all measured electric charges were multiples of 1.6 x 10-19 Coulombs. A droplet could have an excess or deficiency of one or more electrons, but never gain or lose a fraction of an electron. This led to the mass of the electron being determined to be 9.1 x 10-31 kg.
Between 1909 and 1916 the American Robert A. Millikan (b1868, d1953, ←at left) devised increasingly precise ways to demonstrate that there is a smallest possible electric charge and to measure that charge. Earlier Charles Wilson in England had devised what became known as a cloud chamber to visualize the path of charged particles by a trail of droplets. After an observation, the trail of charged droplets could be cleared from the apparatus by applying an electric field arranged by parallel plates similar to what Thomson use. But in air, the tiny droplets quickly reach a terminal velocity, and then travel at constant velocity. The accelerating force on their electric charge quickly is balanced by friction determined by the droplet size. Millikan realized that the experiment could be turned around so by measuring the size and terminal speed, he could calculate the electric charge on each droplet. But condensation or evaporation often changed the tiny droplet size complicating the calculations. While on a train journey to a convention, Millikan glanced at his watch and realized that the clock oil used for long lasting lubrication was chosen for its lack of evaporation! Substituting a fine mist of oil droplets (inside the pot above right→ to assure uniform temperature, block air currents and extraneous electric fields), Millikan demonstrated that all measured electric charges were multiples of 1.6 x 10-19 Coulombs. A droplet could have an excess or deficiency of one or more electrons, but never gain or lose a fraction of an electron. This led to the mass of the electron being determined to be 9.1 x 10-31 kg.
to follow
Finally, record your procedures, measurements, and findings in your journal. If you need course credit, use your observations recorded in your journal to construct a formal report
![]()
next Experiment
to ie-Physics menu
to site menu