![]()
![]()
You may have read in earlier sections how the early Greeks used observations, measurements, and logic to develop successful understanding of the heavens and many earthly phenomena. So it may seem strange that they made wine and used various chemical processes with food, but spent very little effort to understand that chemistry. But in the years before mechanical engines were invented to assist us, the ancient Greeks used slaves to perform menial tasks such as meal preparation. So perhaps thoughts about food and other chemicals was considered only appropriate for their slaves. Activities that are either taken for granted, or not considered worthy of consideration must wait for other minds to investigate and develop understanding.
However the Greeks did ponder, almost from a mathematical perspective, whether the world was completely full of continuous materials or made of discrete, separate pieces. Thus they coined the term atom to discuss separate pieces separated by voids of vacuum. But having difficulty imagining nothingness
at a time when zero hadn't been invented, most Greeks preferred the concept of continuous matter which might be distinguishable into different basic forms called elements. While we use such vocabulary terms today, their current meanings are often far from the original intent.
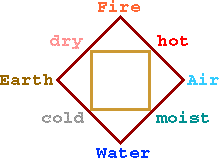 Consistent with their other concepts about the cosmos, Greeks believed that the basic elements should be distinguished by observable differences. So each state of matter, rigid solids, the pourable liquids, elusive vapors, and transforming flames were each considered distinct elements: named earth, water, air and fire respectively. For example, the Greeks thought the element earth to be both dry and cold, while air was considered hot and moist.
Consistent with their other concepts about the cosmos, Greeks believed that the basic elements should be distinguished by observable differences. So each state of matter, rigid solids, the pourable liquids, elusive vapors, and transforming flames were each considered distinct elements: named earth, water, air and fire respectively. For example, the Greeks thought the element earth to be both dry and cold, while air was considered hot and moist.
Today's chemistry does not have the same intellectual roots as other parts of physics. Chemistry arose out of the traditions of metallurgy and medicine, both secret arts rather than intellectual ponderings willingly shared with anyone interested. The traditions that became alchemy in Egypt were gathered from the most successful middle eastern metallurgy and ancient Egyptian medical practices, sprinkled with the Greek vocabulary of elements and the Platonic use of causes
accounting for observed changes. The alchemists made their living by producing potions for the sick and metal products for those who could afford them. To protect their livelihood, their skills were guarded by obscure explanations that were passed to apprentices more by ritual than understood ideas or concepts. So the Greek concept of elements had a different role in chemistry than other Greek concepts played in the development of physics.
Alchemy did not grow and develop with the success of the fledgling Greek scientific methods that guided the development of astronomy as discussed earlier. Unlike astronomy that developed more from a drive to better understand the universe as God made it, alchemy's development was driven to provide more subsistence for the alchemist. Weights of combined ingredients became increasingly a concern to alchemists because it was believed that the successful production of gold and other products depended on the ratio of ingredients. The concept of conservation of mass which became the critical tool forcing a new chemistry actually developed in hope of profit rather than intellectual curiosity.
Only in the 17th and 18th Centuries when the vague concepts and tidbits of knowledge left alchemy and became interesting to curious intellectuals of other occupations did the revolution occur that created modern chemistry. Some of the mystery and magic inherent in the original alchemy still remains a part of modern chemistry.
One of the widely known skills of alchemists was their ability to change base metals into gold. Alchemists thought metals were formed by adding precisely correct quantities of substances and fire to elemental earth. It should be possible to reproduce this successes of alchemy, starting with common modern materials, transform a less valued metal by adding more desirable properties.
Materials:Finally, record your procedures, measurements, and findings in your journal. If you need course credit, use your observations recorded in your journal to construct a formal report
![]()
next Experiment
to ie-Physics menu
to site menu