![]()
![]()
 Fluorescing minerals and photographic film provide Röntgen and Becquerel methods for detecting radiation later understood to carry large quanta (bundles) of energy. But neither method provided much information about the radiation itself. Ernest Rutherford (1871-1937, at left) was born and raised in New Zealand but appreciated that the center of fundamental research was on the back side of the earth in Europe. Using a scholarship to Cambridge University, Rutherford embarked on an career involving a number of simple but creative techniques to identify the nature of this high energy radiation and the structure of the atoms from which it comes. In the end many considered Rutherford the greatest experimental scientist of the Century!
Fluorescing minerals and photographic film provide Röntgen and Becquerel methods for detecting radiation later understood to carry large quanta (bundles) of energy. But neither method provided much information about the radiation itself. Ernest Rutherford (1871-1937, at left) was born and raised in New Zealand but appreciated that the center of fundamental research was on the back side of the earth in Europe. Using a scholarship to Cambridge University, Rutherford embarked on an career involving a number of simple but creative techniques to identify the nature of this high energy radiation and the structure of the atoms from which it comes. In the end many considered Rutherford the greatest experimental scientist of the Century!
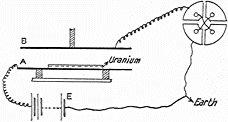 In 1899 Rutherford used an electrometer (shown in his diagram at right) as developed by the Curies to detect radiation. Rutherford noted that the radiation from uranium which caused electric charge to leak away could be blocked by covering with increasing thicknesses of aluminum. But after much of the radiation was blocked, additional aluminum reduced the remaining radiation much more gradually. Rutherford concluded that uranium must emit two distinct types of radiation with differing penetrating powers. Using the Greek alphabet, Rutherford labelled these α (pronounced
In 1899 Rutherford used an electrometer (shown in his diagram at right) as developed by the Curies to detect radiation. Rutherford noted that the radiation from uranium which caused electric charge to leak away could be blocked by covering with increasing thicknesses of aluminum. But after much of the radiation was blocked, additional aluminum reduced the remaining radiation much more gradually. Rutherford concluded that uranium must emit two distinct types of radiation with differing penetrating powers. Using the Greek alphabet, Rutherford labelled these α (pronounced alpha
), and β (pronounced beta
). (Read Rutherford's, Uranium Radiation and the Electrical Conduction, from the Philosophical Magazine, January 1899) β radiation has the characteristics of fast electrons. In 1900 the French physicist Paul Villard (1860-1934) found an even more penetrating type of radiation which was called γ (pronounced gamma
). Villard recognized γ has the properties of X-rays. (The difficulty identifying α radiation will be a topic of Experiment VI-4.)
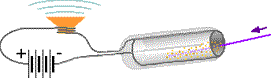 Hans Geiger (1882-1945), while working as an assistant to Rutherford in 1908, developed a device which uses electrical current through a gas ionized by radiation as a signal to count each passage of radiation. Geiger and Walter Müller (1905- ) improved the detector in 1928. The Geiger counter uses the radiation as a trigger for the electrical signal. Radiation enters the Geiger-Müller tube through a very thin (mica) window at one end (see diagram at left.) or in other tubes along one side. Molecules of a gas inside the tube are torn apart forming electrically charged ions either by electromagnetic collisions of γ (gamma) rays with the molecules or by strong magnetic fields from passing α (alpha) or β (beta) radiation. Unlike the originally uncharged gas molecules, the ions carry electric charge across the gap between a central metal wire and the metal cylinder. A high voltage power source pumps replacement charge to the metal cylinder and wire. If the wires from the power supply to one of the electrodes pass around a speaker magnet, an audible click occurs each time the radiation enters the tube, ionizes the gas, and generates a surge of electric current through the wires. A chemical reaction rapidly regenerates the original molecules so the tube will be ready to detect the next entering radiation.
Hans Geiger (1882-1945), while working as an assistant to Rutherford in 1908, developed a device which uses electrical current through a gas ionized by radiation as a signal to count each passage of radiation. Geiger and Walter Müller (1905- ) improved the detector in 1928. The Geiger counter uses the radiation as a trigger for the electrical signal. Radiation enters the Geiger-Müller tube through a very thin (mica) window at one end (see diagram at left.) or in other tubes along one side. Molecules of a gas inside the tube are torn apart forming electrically charged ions either by electromagnetic collisions of γ (gamma) rays with the molecules or by strong magnetic fields from passing α (alpha) or β (beta) radiation. Unlike the originally uncharged gas molecules, the ions carry electric charge across the gap between a central metal wire and the metal cylinder. A high voltage power source pumps replacement charge to the metal cylinder and wire. If the wires from the power supply to one of the electrodes pass around a speaker magnet, an audible click occurs each time the radiation enters the tube, ionizes the gas, and generates a surge of electric current through the wires. A chemical reaction rapidly regenerates the original molecules so the tube will be ready to detect the next entering radiation.
While Rutherford used an electroscope, we can use the results of a Geiger counter to investigate the difference in radiation intensity due to shielding first noted by Rutherford. Despite the possibility of students working safely with radiation, the availability of radiation sources seems to be declining. Because you probably don't have both a Geiger counter and appropriate sources of radiation, electrical signals from a Geiger counter have been stored via an audio file so you can listen to clicks from a Geiger counter and measure the radiation intensity by counting the number of clicks per minute. Rather than being immediately played through a speaker at the time of the experiment, the stored signal may be played through your computer's speakers at your convenience. The Geiger counter does not capture the energy of the radiation, but instead uses much more power drawn from an electrical power supply, triggered by the radiation. Likewise none of the original radiation is contained in the audio file, but rather the energy released from your speakers comes from the same power source that runs your computer, triggered by the information stored in the audio file. Compared to Becquerel's experiment, using the Geiger counter greatly shortens the time and effort needed to detect radiation and allows us to more accurately quantify the intensity of the radiation.
