![]()
![]()
 While William Gilbert summarized the properties of magnetics and electricity in 1600, he did not believe that electric bodies could repel. But in 1646 Sir Thomas Browne published an account of electrical repulsion. A century later the repulsion of two negative charges proved difficult for Benjamin Franklin's proposal that only one kind of fluid exists. How could two bodies experience electrical repulsion if Franklin claimed they were deficient of electric fluid?
While William Gilbert summarized the properties of magnetics and electricity in 1600, he did not believe that electric bodies could repel. But in 1646 Sir Thomas Browne published an account of electrical repulsion. A century later the repulsion of two negative charges proved difficult for Benjamin Franklin's proposal that only one kind of fluid exists. How could two bodies experience electrical repulsion if Franklin claimed they were deficient of electric fluid?
About 1775 Franklin noted a small cork hanging outside an electrically charged metal can was strongly attracted, but when lowered by a thread inside the can, the cork experienced no force. Franklin had difficulty understanding why the cork did not experience a force especially when located inside but very near the charged metal can. The protestant clergyman Joseph Priestley (1733-1804 at left), whose destiny was changed by meeting Franklin in London, repeated the experiment. Recalling that Newton had written that because of the inverse square force law there would be no gravitational force inside a hollow planet, Priestly surmised by analogy that a similar inverse square law must apply for electric force. The French physicist Charles Augustin de Coulomb (1736-1806 at right) invented a delicate torsion balance and used it to provide experimental verification of such an inverse square law.
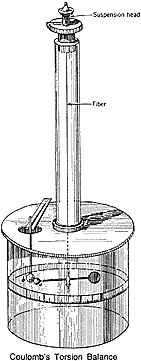
Coulomb's balance (shown here) used a pair of spheres suspended by a long, easily twisted fiber inside a shielded case. When another charged sphere was nearby, the fiber supporting the spheres was slightly twisted. To vary the amount of charge, an identical uncharged sphere was touched to one of the charged spheres. The electric fluid was shared equally between the two spheres, resulting in half the original charge on each sphere.
Today scientists often need to use equipment beyond their personal ability to build or purchase. Research institutions around the world used government and other grant monies to build such equipment. The institutions either host scientists selected to perform proposed experiments, or the institution support staff conducts the proposed experiment and supplies the results to the scientists who proposed the research. It is not so much that the expense is too great or that equipment such as Coulomb created is too difficult to make. Rather perfecting the apparatus and gathering usable data requires great patience and care. Over several decades, students in the author's classes have experienced much difficulty gathering suitable data. Therefore data from more successful attempts has been gathered and provided in the table below:
Procedure:
| Separation distance (cm) |
1 / d (cm-1) |
1 / d2 (cm-2) |
Electrical Force (∝ deflection from ⊥ in cm) |
| 0.3 | 11 | ||
| 0.4 | 9.0 | ||
| 0.7 | 7.4 | ||
| 1.0 | 6.6 | ||
| 1.2 | 5.8 | ||
| 1.6 | 5.2 | ||
| 2.0 | 4.6 | ||
| 2.4 | 4.0 | ||
| 3.1 | 3.7 | ||
| 3.6 | 3.2 | ||
| 4.3 | 2.9 | ||
| 4.7 | 2.8 |
Finally, record your procedures, measurements, and findings in your journal. If you need course credit, use your observations recorded in your journal to construct a formal report
![]()
to next Experiment
to ie-Physics menu
to site menu