![]()
![]()
That solidity, liquidity, and aeriform elasticity, are only three different states of existence of the same matter, or three particular modifications which almost all substances are susceptible of assuming successively, and which solely depend upon the degree of temperature to which they are exposed; or, in other words, upon the quantity of caloric with which they are are penetrated. Antoine Lavoisier translated from his 1789 Traité élémentaire de Chemie...
If a vapor such as steam is cooled sufficiently, it condenses to a liquid. And if that liquid is cooled sufficiently, it will eventual freeze into a solid. Those transformations can be reversed by adding energy in the form of heat (which Lavoisier called caloric). But why does that happen? What occurs on the atomic and molecular scale that results in these physical phase changes we observe with our senses?
Apparently several forces are at work. Chemists have suspected since the early 1800s that strong electrical forces hold clusters of atoms together in fixed proportions to form molecules. Such strong electrical forces, called chemical bonds, hold two Hydrogen atoms (symbol H) together with an Oxygen atom (O) forming each molecule of water (symbolized as H2O). These water molecules generally stay together as integral units, whether in steam, liquid water, or ice. Only weaker bonds between molecules broke when warmed ice melted or heated liquid water boiled.
Nineteenth century chemists had assumed that some atoms pull electric charge from other atoms, resulting in ions with positive and negative charges. These oppositely charged ions then had strong electrical attraction. But until the nature of this electrical exchange was investigated further in the closing years of the 19th century, such ionic bonds were not well understood. Investigators found that what Benjamin Franklin labelled negative electrical charge could be extracted from a wide variety of substances. Careful measurements found that this negative charge was carried by tiny pieces of atoms which were identical for all substances. These negatively charged pieces of atoms were eventually called electrons. 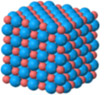 The transfer of electrons forming positive and negative charged ions nicely explains the chemical bonds in many substances such as table salt, NaCl. In the chemical reaction between the very reactive metal Sodium, Na, and the very reactive poisonous gas Chlorine, Cl, each Chlorine atom pulls one electron away from a Sodium atom, resulting in electrically negatively charged Chloride ions, Cl– and positively charged Sodium ions, Na+. The oppositely charged ions with strong electrical attraction form three dimensional crystal lattices composed of layers of alternately placed Sodium (here + is colored red) and Chloride (– is colored blue) ions.
The transfer of electrons forming positive and negative charged ions nicely explains the chemical bonds in many substances such as table salt, NaCl. In the chemical reaction between the very reactive metal Sodium, Na, and the very reactive poisonous gas Chlorine, Cl, each Chlorine atom pulls one electron away from a Sodium atom, resulting in electrically negatively charged Chloride ions, Cl– and positively charged Sodium ions, Na+. The oppositely charged ions with strong electrical attraction form three dimensional crystal lattices composed of layers of alternately placed Sodium (here + is colored red) and Chloride (– is colored blue) ions.
 But other molecules such as the diatomic Nitrogen, N2, and Oxygen, O2, found in air could not be explained by ionic bonds. Such molecules were not understood until Gilbert N. Lewis (b1875, d1946, photo right→) proposed in 1916 that such molecules might be bonded together by sharing of electron pairs between atoms. Irving Langmuir (b1881, d1957) suggested in 1919 that bonds produced by sharing electrons be called covalent.
But other molecules such as the diatomic Nitrogen, N2, and Oxygen, O2, found in air could not be explained by ionic bonds. Such molecules were not understood until Gilbert N. Lewis (b1875, d1946, photo right→) proposed in 1916 that such molecules might be bonded together by sharing of electron pairs between atoms. Irving Langmuir (b1881, d1957) suggested in 1919 that bonds produced by sharing electrons be called covalent.
 Linus Pauling (b1901, d1994, ←photo left) noted that many molecules seem to exhibit a combination of ionic and covalent bonding. In 1932 Pauling proposed electronegativity as a way to calculate the proportion of each. (The differences between ionic and covalent bonding will become more significant in the next investigation, P6, solutions.)
Linus Pauling (b1901, d1994, ←photo left) noted that many molecules seem to exhibit a combination of ionic and covalent bonding. In 1932 Pauling proposed electronegativity as a way to calculate the proportion of each. (The differences between ionic and covalent bonding will become more significant in the next investigation, P6, solutions.)
Generally ionic and covalent bonds hold molecules firmly together unless very high temperatures are reached. It is usually a collection of other, weaker forces which give way when temperature is increased and we witness phase changes such as melting, boiling, condensing and freezing:
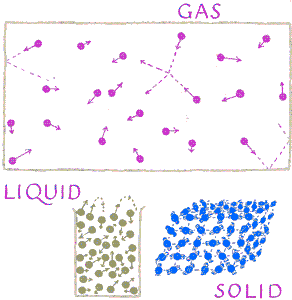 Chemists typically find the explanations using what we experience with our senses when we try to understand what atoms are doing. All atoms are moving. Their average speed is determine by their absolute temperature (that is, on a scale which starts with zero as the theoretically coldest possible). Generally warmer atoms have more kinetic energy so move faster. But the chemical bonds between atoms often restrict their motion. In solids each atom is locked into an approximate position and only allowed to vibrate around that location. In some large molecules the atoms are help in long chain-like structures which are so tangled as to maintain their positions. In other crystalline solids the atoms are arranged in three dimensional lattices. But unless there are flaws such as vacancies (lattice
Chemists typically find the explanations using what we experience with our senses when we try to understand what atoms are doing. All atoms are moving. Their average speed is determine by their absolute temperature (that is, on a scale which starts with zero as the theoretically coldest possible). Generally warmer atoms have more kinetic energy so move faster. But the chemical bonds between atoms often restrict their motion. In solids each atom is locked into an approximate position and only allowed to vibrate around that location. In some large molecules the atoms are help in long chain-like structures which are so tangled as to maintain their positions. In other crystalline solids the atoms are arranged in three dimensional lattices. But unless there are flaws such as vacancies (lattice holes
missing atoms from possible locations) in the structures, the atoms are not allowed to change positions, locked in by neighbors close by on all sides. (Such vacancies are of critical importance in solid state batteries and solid state electronics, topics for later study.)
In liquids the atoms are nearly as tightly packed together as they are in solids. But the forces holding a molecule to its neighbors is weak enough to allow the motion of each to break away from neighbors and to tumble and gradually squeeze past. This results in the ability of liquids to flow as a fluid and mix.
In gases the forces between molecules is so weak compared to their random motion that when bouncing off each other, they push apart, creating significant empty space between themselves. This empty space between molecules results in gases having much lower densities than either solids or liquids. This also explains why gases are highly compressible, and when not restricted, capable of expanding indefinitely.
As solids are heated, the atoms move faster resulting in collisions with the neighbors being more violent. Eventually if heating is continued the force of the collisions become strong enough to break the bonds holding molecules to their neighbors and to force enough room between them to squeeze to new locations. We call this transition from solid properties to the flow of liquid melting.
You may have noticed that the description above of atoms in solids being surrounded on ALL sides pertains to the vast majority of atoms in solids. But the rare atoms occupying the surfaces of the solid are obviously not fully surrounded. Here on a surface, edge, or corner, the departure of a molecule is not blocked. So depending on the temperature and vibration of the atoms, a few molecules might through random collisions with their neighbors occasionally obtain enough motion to actually break away from the solid. This process is known as sublimation. You may have noticed it when, despite temperatures always below freezing, an old snowfall gradually disappears. It is also the reason that ice cubes in an unused tray in the freezer gradually shrink, or moisture gradually escapes from meat and other foods left in well sealed packages in a freezer. The process also occurs exclusively when the surrounding atmospheric pressure is reduced below what will be explained below as the triple point.
If a liquid is cooled, the process of melting is reversed and the weak forces between molecules become sufficient to lock molecules into lattice positions when random motions by chance moves them into those locations. The process has long been called freezing.
If the liquid is heated sufficiently, the increased kinetic energy eventually results in molecules rebounding off neighbors with enough momentum to drive the molecule and its neighbor apart. Where this begins to occur in the body of the liquid, other nearby molecules find there is now an open surface allowing the easier process of sublimation to occur. The result is usually a rapid increase in the number of molecules moving about the empty space, creating a bubble. This process we call boiling proceeds when the vapor pressure building up inside the bubbles exceeds the external pressure exerted on the liquid. The bubbles expand and because of their lower density, float to the top of the liquid. If the external pressure is greater (say in a part of the liquid away from the heat), the molecules in the surrounding liquid are pushed back together, providing for the observation of bubbles collapsing.
With increased in pressure on a gas, or a decrease in temperature of the gas, the weak forces between molecules can begin to restrain the molecules' previous free motions and elastic collisions, resulting in inelastic collisions and the formation of a liquid. The chemical bonds are sufficiently strong to capture the colliding molecules and restrain them from rebounding. This is the process of condensation such as occurs when a cloud forms and it rains. The energy captured in the inelastic collisions often results in an increase in ambient temperature.
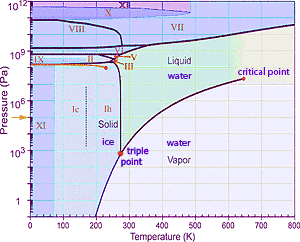 Sometimes there is value in constructing a graph of a substance's vapor pressure verses temperature (such as the phase diagram for water at right→). Curves distinguish conditions which allow the existence of solid, liquid, and gas phases for the particular substance. Crossing one of these lines results in phase change as described just above. But several specific locations are apparent on such a graph. There is a point with particular temperature and vapor pressure know as the triple point where solid, liquid, and gaseous (= vapor) phases of the substance can co-exist in equilibrium. There is also a critical point of extreme pressure beyond which the distinction between gas and liquid ceases to exist; molecules in the gas are squeezed so close together that no empty space remains, just as in the liquid phase. It might be noted that many substances utilize several different lattice arrangements depending on temperature and pressure conditions. For such variations in the structure of the solid, additional phase transition lines may be drawn on the phase diagram. (Such different lattice structures for ice are distinguished by Roman numerals on this graph. There is also a gold arrow → on the graph at the pressure equal to one atmosphere. If you follow horizontally from that arrow, you might note that under that pressure water melts at 273K and boils at 373K.)
Sometimes there is value in constructing a graph of a substance's vapor pressure verses temperature (such as the phase diagram for water at right→). Curves distinguish conditions which allow the existence of solid, liquid, and gas phases for the particular substance. Crossing one of these lines results in phase change as described just above. But several specific locations are apparent on such a graph. There is a point with particular temperature and vapor pressure know as the triple point where solid, liquid, and gaseous (= vapor) phases of the substance can co-exist in equilibrium. There is also a critical point of extreme pressure beyond which the distinction between gas and liquid ceases to exist; molecules in the gas are squeezed so close together that no empty space remains, just as in the liquid phase. It might be noted that many substances utilize several different lattice arrangements depending on temperature and pressure conditions. For such variations in the structure of the solid, additional phase transition lines may be drawn on the phase diagram. (Such different lattice structures for ice are distinguished by Roman numerals on this graph. There is also a gold arrow → on the graph at the pressure equal to one atmosphere. If you follow horizontally from that arrow, you might note that under that pressure water melts at 273K and boils at 373K.)
 Support an ice cube so apparatus can hang down underneath.
Support an ice cube so apparatus can hang down underneath.![]()
to next experiment: Solutions
to menu for Nanochemistry, Colloidal & Physical Chemistry
to ie-Chemistry menu
to site menu