![]()
![]()
 Luminescence (Latin, lumen = light) is the re-emission of light from an object upon being excited by another light or energy source. Luminescence can be divided into two phenomenon: phosphorescence and fluorescence. A phosphorescent body continues to emit light even after it is no longer being excited. A fluorescent body stops emission almost immediately after the excitation stops. In both forms of luminescence the chemical substance absorbs short wave light energy and emit it somewhat longer wavelength light: for example, a florescent material might absorb invisible, short-wave, high-energy such as ultraviolet light or X-rays, then emit light of less energy and greater wavelength in the visible range making the fluorescing color seem extraordinarily bright. When a chemical reaction provides the excitation energy, the process is often called chemoluminescence. One example is luminol, which reacts with H2O2 (hydrogen peroxide) in the presence of a catalyst to emit bluish green light. This is sometimes used by police departments to detect traces of blood which serves as an effective catalyst for the reaction. One subtype of chemoluminescence is bioluminescence, processes where chemical reactions inside living creatures such are fireflies and sea algae produce light either on their own or with the help of symbionts. Other types of luminescence include electro and carthodoluminescence. Electroluminescence involves excitation by an electrical current, such as in the case of light emitting diodes. Carthodoluminescence is achieved by electron bombardment as in a cathode ray tube TV screen.
Luminescence (Latin, lumen = light) is the re-emission of light from an object upon being excited by another light or energy source. Luminescence can be divided into two phenomenon: phosphorescence and fluorescence. A phosphorescent body continues to emit light even after it is no longer being excited. A fluorescent body stops emission almost immediately after the excitation stops. In both forms of luminescence the chemical substance absorbs short wave light energy and emit it somewhat longer wavelength light: for example, a florescent material might absorb invisible, short-wave, high-energy such as ultraviolet light or X-rays, then emit light of less energy and greater wavelength in the visible range making the fluorescing color seem extraordinarily bright. When a chemical reaction provides the excitation energy, the process is often called chemoluminescence. One example is luminol, which reacts with H2O2 (hydrogen peroxide) in the presence of a catalyst to emit bluish green light. This is sometimes used by police departments to detect traces of blood which serves as an effective catalyst for the reaction. One subtype of chemoluminescence is bioluminescence, processes where chemical reactions inside living creatures such are fireflies and sea algae produce light either on their own or with the help of symbionts. Other types of luminescence include electro and carthodoluminescence. Electroluminescence involves excitation by an electrical current, such as in the case of light emitting diodes. Carthodoluminescence is achieved by electron bombardment as in a cathode ray tube TV screen.
Chinese books were written about fluorescence and phosphorescence as early as 1500 B.C.
Luminous materials were known in the Western cultures by the time of the Greeks and Romans. Aristotle wrote about such effects observed in the sea, meat and rotting wood.
In 1603 Vincenzo Casciarolo, a shoemaker and alchemist in Bologna, discovered by accident a phosphor known as the Bolognian stone (also called Bolognian phosphor) which glows after exposure to light. The stone composed of barium sulphide became luminous when put between red-hot coals. Galileo Galilei (b1564, d1642), an Italian scientist, wrote of the Bolognian stone: It must be explained how it happens that the light is conceived into the stone, and is given back after some time, as in childbirth.
(above right, The Alchymyst by Joseph Wright.)
 The German Jesuit priest Athanasius Kircher (←at left, b1601 in Buchonia along the Upper Rhone, d1680 in Rome), among his numerous achievements, wrote a book in 1646 called Ars Magna Lucis et Umbrae in which he described an interesting observation of the wood extract Lignum nephriticum. Light passing through an aqueous infusion of this wood appeared more yellow while light reflected from the solution appeared blue. The blue light is an result of fluorescence. Kircher also discussed the application of fireflies to illuminate houses, an application fo bioflorescence.
The German Jesuit priest Athanasius Kircher (←at left, b1601 in Buchonia along the Upper Rhone, d1680 in Rome), among his numerous achievements, wrote a book in 1646 called Ars Magna Lucis et Umbrae in which he described an interesting observation of the wood extract Lignum nephriticum. Light passing through an aqueous infusion of this wood appeared more yellow while light reflected from the solution appeared blue. The blue light is an result of fluorescence. Kircher also discussed the application of fireflies to illuminate houses, an application fo bioflorescence.
In 1674 Christoph Adolph Balduin (b1632, d1682) first produced calcium nitrate. Everything in a glass vessel after being highly heated and dried was found to be luminous. Named Balduin's phosphorus (phosphorus = carrier of light), such minerals are stimulated to phosphorescence by a prior exposure to radiation such as sunlight. Emitting a fairly bright light, they were looked upon as a magnet or a kind of sponge which could suck up light and give it out again. Similarly Dr. Brand attempted in 1674-5 to distil human urine and discovered the element Phosphorus, named for its delayed light readmission.
In 1842, Sir George Gabriel Stokes (b1819, d1903, at right→) described the luminescence observed in the fluorspar mineral (calcium fluoride) as fluorescence. Stokes, professor of mathematics and physics at Cambridge, noted in 1852 that the fluorescent light is always of a longer wavelength than the exciting light. The following year Stokes coined the term fluorescence from the term "internal dispersion."
Alexandre-Edmond Becquerel (b1820, d1891, second son of physicist Antoine Becquerel, father of physicist Henri Becquerel) showed that nearly all fluorescent substances can also be phosphorescent although in some cases the phosphorescence may continue for only a fraction of a second.
By 1908 Heinrich Knoen, a German physicist, alphabetically listed 1700 known fluorescent compounds.
All types of luminescence are the result of electron behavior inside atoms. And that understanding did begin until the second decade of the 20th Century.
 In 1913 Niels Bohr (←at left, b1885, d1962) proposed an atomic model, assuming arbitrary orbits now abandoned, where electrons were only permitted to have certain quantized energies. The energy aspects of his theory formed the basis for our current understanding. Electrons normally occupy the lowest available (
In 1913 Niels Bohr (←at left, b1885, d1962) proposed an atomic model, assuming arbitrary orbits now abandoned, where electrons were only permitted to have certain quantized energies. The energy aspects of his theory formed the basis for our current understanding. Electrons normally occupy the lowest available (ground
) energy levels. But if a matching bundle of energy should come by, an electron could absorb exactly the needed energy and be excited to a higher permitted energy level.
By absorbing a photon of visible light carrying the energy matching that an electrons needs to be excited to a higher energy level, the electron absorbs that light and by subtracting that light from the total available, makes the material appear colored. This process of selective light absorption gives color to many of the materials we encounter in our lives. But such excited electron states are inherently unstable, so the excited electron will soon return to its normal ground state (So), typically releasing the energy as heat. But the energy could be released as a bundle of light identical to the original's energy and color. If the electron was excited several energy levels, the electron could step down one energy level at at time, emitting appropriate smaller bundles of light with longer wavelengths and relatively redder colors.
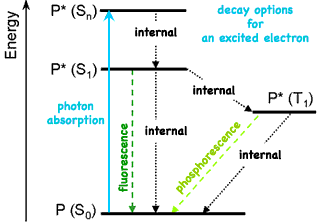
There are several options for the unstable excited electron to release energy. Using vibrations, it can internally shift some or all the energy inside the atom (eventually releasing it as heat), simply re-emit all the energy as light, or decline to a lower permitted energy state by emitting the energy difference as light.
 The electron has some of its inherent energy/mass embedded in a quantized property (first proposed in 1925 by Ralph Kronig, George Uhlenbeck, and Samuel Goudsmit) called spin which is responsible for the electron's inherent magnetic moment, its fundamental magnetic field. (While the name originated because a spinning electrically charged body does create a magnetic field, this is inherent magnetic moment and cannot be due to actual spinning of the electron! Electrons are essential zero sized points in space, and even if a reasonable size were assigned the electron, it would need to spin faster than the speed of light to generate sufficient magnetic moment.) Based on its properties, the electron is assigned a spin of 1/2. According to the statistics of Enrico Fermi and Paul Dirac, the wave functions of such
The electron has some of its inherent energy/mass embedded in a quantized property (first proposed in 1925 by Ralph Kronig, George Uhlenbeck, and Samuel Goudsmit) called spin which is responsible for the electron's inherent magnetic moment, its fundamental magnetic field. (While the name originated because a spinning electrically charged body does create a magnetic field, this is inherent magnetic moment and cannot be due to actual spinning of the electron! Electrons are essential zero sized points in space, and even if a reasonable size were assigned the electron, it would need to spin faster than the speed of light to generate sufficient magnetic moment.) Based on its properties, the electron is assigned a spin of 1/2. According to the statistics of Enrico Fermi and Paul Dirac, the wave functions of such spin 1/2 particles
can't occupy the same space with identical particles, but can pair with the wave function of a particle of opposite spin to occupy the same space. (When then do so they are said to be in the singlet state, S, because of the single spectral line they generate, even in a magnetic field.) But while an excited electron is unpaired, it can flip to an opposite spin so that its spin is now identical to its original partner. (They are said to be in the triplet state, T, because in a magnetic field their three closely spaced but distinct energy levels produce three hyperfine spectral lines.) But once in this situation, the excited electron cannot rapidly emit its excess energy because that would place two identical electrons in the same space. So only slower processes involving spin reversal are permitted. (Spin is describes as conserved. A spontaneous spin change is forbidden unless coupled with another spin conserving change. At excited energies the probability of violating a forbidden transition is no longer absolutely zero, but remains tiny and rare.)
Each energy releasing decay is characterized by its own rate constant and each excited state is characterized by its lifetime τ. Because sometimes there is a pronounced delay of emission of light from electrons triplet state, that process was given a distinguishing name long before the process was understood. The rapid (~10 ns) re-emission of light when spin is not changed is called fluorescence while the delayed (ms to hours) release accompanying a change in spin is called phosphorescence. If the lifetime of the excited state is long enough, the excited molecule may have time to react with a neighboring molecule.
Since fluorescent and phosphorescent materials are all around us, it ought to be possible to experimentally investigate these phenomena.
| substance | florescent color |
| Mustard made into a solution and then dried | Green |
| Hen's egg - brown fresh | Brilliant scarlet |
| Quinine detected weak solutions | Electric blue |
| Sodium salicylate | Like a star |
| Tinea - ringworm | Bright metallic green |
| Squamous cell carcinoma | Glows like hot coals |
| Secretions of the skin, fingers and nails | Delicate blue |
| Urine | Pale blue |
| Teeth | Bright white |
| Decay, plaque or false teeth | No fluorescence |
| Seborrheic eczema | Dull brownish-yellow |
| Psoriasis (on underside of scales) | Silver white light, (pink) |
| Paraffin wax + paraffin oil | Blue |
| Fluorspar | Blue |
| Alcoholic solution of chlorophyll | Red |
| Crystalline lens of eye under suitable conditions | Bright blue |
Ultraviolet light sources are moderately inexpensive and available in many communities. These are more expensive than visible lamps because they are enclosed by more expensive materials than glass. Normal glass is a strong absorbers of ultraviolet light so unsuitable for being an enclosure. However quartz glass is more transparent to ultraviolet light.
The Sun emits ultraviolet light which also causes suntans, sunburn and occasionally triggers skin cancer. So if a special ultraviolet lamp is not available or affordable, it might be possible to use sunlight as a source.
Caution: Ultraviolet light can damage eyes. Avoid looking directly at a source of such light or at strong reflections such as from a mirror or other shiny surface.
![]()
to next experiment: Capturing Light Energy
to Nanochemistry menu
to e-Chemistry menu
to site menu