![]()
![]()
Much emotional concern surrounds this topic. This investigation attempts to provide a scientific basis for some of those concerns while providing some relevant information and some experimental techniques that may help rationally develop eventual solutions.
The human civilizations on earth have for several millennia developed increasingly using the expenditure of organic molecules as fuel. Early civilizations used wood and as a result deforested large areas leaving some areas now barren. Later civilization used peat, coal, petroleum, and natural gas. While the earth's supplies of these resources have not yet been totally depleted, a global inventory of those resources suggest that depletion is not long in the future. But more importantly, the burning of fossil fuels now seems to be causing a significant annual increase in the previously small amount of carbon dioxide, CO2, in the atmosphere. This gas is known to have a greenhouse effect, allowing passage of visible sunlight, while absorbing longer wave length infrared radiation. This in turn seems likely to increase the average temperature of the earth's surface precipitating climate change predicted to include significant melting of the earth's polar ice caps. Thus the future of human civilization is in jeopardy both due to the depletion of fuels needed to power civilization, but also climate changes and subsequent consequences which have not yet been accurately predicted.
A variety of alternatives have been proposed with different chances of success and hope for addressing the problem.Many countries believe that replacement of the use of fossil fuel for generating electricity with nuclear power plants could significantly help. But fear of the effects of an envisaged accident and long term effects imagined from the release of radioactive isotopes cause many Americans and some Europeans to reject that option. Reliance of the less reliable solar energy collectors and wind turbines remain the only other large scale options available in the near future. Some locations also have geothermal, tidal, or river resources which could help a little. (Solutions need to take into consideration the difficulties of night, periods of calm, and environmental effects of dammed bodies of water and such.)
It has been proposed that Hydrogen, H2, is a cleanly burning fuel that does not produce carbon dioxide and thus would not cause climate change. But Hydrogen is not a fuel that exists in any natural abundance on earth. Using fossil fuels to create Hydrogen, due to inefficiencies in conversion, would generate larger amounts of carbon dioxide that would direct usage of the fossil fuels. Generating Hydrogen from water using electricity created from nuclear power plants, wind turbines or solar collectors will likely cost at least an order of magnitude (10x) more than petroleum.
One option being developed by Brazil is to produce ethanol, (ethyl alcohol) CH3CH2OH, produced from sugar cane at a cost of less than $1 per gallon. (Ethanol production from corn in the United States is not as economical.) Use of ethanol or similar organic molecules from rapidly growing and easily converted crops has an advantage that might not be immediately noticed. While burning ethanol does produce carbon dioxide, unlike the burning of fossil fuels, it doesn't add to the total because it is essentially recycled by the next crop grown to produce the next batch of ethanol.
To compare the energy in different fuels, the heat produced should be divided by the amount of fuel consumed.
If you are a bit uncertain of the distinctions between heat, energy, and temperature, this is a good time to make clarifications: Long before these three things were understood, people who cooked food and pottery developed tools to measure the temperatures of their ovens and kilns. Only in the mid-nineteenth century was the Kinetic Molecular Theory developed explaining that temperature was proportional to the average kinetic energy of the molecules, i.e., the higher the temperature, the faster molecules moved or vibrated. Every moving object, be it a molecule or much larger, has as a result of its state of motion this kinetic energy = 1/2 mv2 where v it the object's velocity. Physicists realized that calculated properties were most useful if they could be defined so that they were conserved, that is, never created nor destroyed. Clearly kinetic energy is not conserved because it decreases when the object slows. But physicists were careful to define other kinds of energy so that to increase an object's velocity would require an equal amount of some other energy be transferred into the object. Likewise to slow an object's motion, the necessary energy would need to be transferred elsewhere. One other form of carefully defined energy was heat, related to temperature by the above equation. Other forms of energy such as sound and light were consistently defined. Energy could also be stored in forces such as the chemical bonds between atoms or in gravity when an object thrown upwards slows only to regain speed as it falls back downward. This group of carefully defined forms of energy has proven to be one of the most valuable ideas every invented by humans!
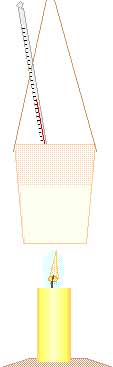
Communicating technical information such as observations and findings is a skill used by scientists but useful for most others. If you need course credit, use your observations in your journal to construct a formal report.
![]()
to Physical Chemistry menu
to e-Chemistry menu
to site menu