![]()
![]()
Ozone is often a harmful constituent in air pollution found in urban areas. While Ozone is an important part of the upper atmosphere where it protects ground level life from harmful radiation, it is not a regular part of the atmosphere at ground level. Rather is is created in the lower atmosphere by a sequence of chemical reactions involving sunlight and other air pollutants.
Volatile organic compounds (VOCs) are regarded as precursors of ground level Ozone, O3 by an indirect process. It is believed that the reactive hydroxyl radical, OH, with Oxygen, O2, first reacts with VOCs and carbon monoxide:
where RO2 represents where the hydrogen in an organic molecule, represented generically by R, has been replaced by the oxygen molecule. For example butane might react with the hydroxyl radical to produce butyl peroxy radical and water.
The initiating reaction above could be followed by the conversion of NO present in polluted air with either of the two radicals:
This regenerates the OH radical and, using the example of the butane, synthesizes the secondary VOC, butionaldehyde
In the final step, NO2 is decomposed by sunlight producing very reactive atomic oxygen, O, which combines with molecular oxygen, O2, to create the corrosive ozone, O3. This requires collision with another molecule to carry away energy which would otherwise result in the Ozone immediately decaying:
The ground-level ozone causes lung irritation and damage in animals. It can aggravate asthma, reduce lung capacity, and increase the chance of pneumonia and bronchitis. Ozone also causes similar damage to plants and some cases leads to the death of certain types of plants. Ozone also damages paint and other surfaces.
Chemists refer to substances such as normal Oxygen, O2, and Ozone, O3, as allotropes. These are composed of atoms of the same element but in different structural arrangements. As a result they have different physical properties and often different chemical properties as well.
Because Ozone is a strong oxidizer, it is corrosive to a number of substances including natural rubber. Therefore a simple tan colored rubber band can be used as an inexpensive detector of Ozone. Natural rubber is a long zig-zag chain of hydrocarbon with double bonds between the carbons every four bonds. The zig-zag shape allows the rubber to stretch by bending the bonds from their ideal angle a bit. But these bent bond become more reactive to oxidation by ozone. So while a natural rubber band might exist for years unstretched in a drawer, while stretched how long it lasts depends on the amount of ozone present.
Because stretched rubber is easily oxidized by Ozone, manufacturers add Carbon (black graphite or soot, or other colored substances) to tires to preferentially react with Ozone thus preventing the rapid aging of the stretched rubber.
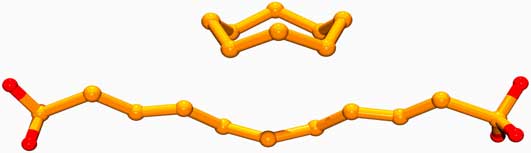
It is difficult to investigate the gaseous allotropes of Oxygen. However it is easier to investigate some of the allotropes of the next family member immediately below Oxygen on the periodic chart, Sulfur. At room conditions Sulfur normally exists as a yellow solid composed of a crown shaped ring of S8 (shown below). But gentle heating results at 113°C in the rings breaking, melting into S16 chains with an orange color (also below). Further heating results about 180°C in the formation of viscous (thick) brown polymer with on the order of a million atoms per molecule. Rapid cooling results in the formation of an amorphous, rubbery solid of this polymer. But this is unstable so that eventually the yellow S8 solid reforms.
This experiment requires good ventilation. Either work outside where breezes will carry away offensive fumes or under a hood that vents outside where fumes will not create problems for others. Fire danger: Work with a small amount of Sulfur. Heated Sulfur occasionally ignites, burning with Oxygen with a blue flame to form SO2, a choking, toxic gas.
Communicating technical information such as observations and findings is a skill used by scientists but useful for most others. If you need course credit, use your observations in your journal to construct a formal report.
Because Sulfur is a minor component in many proteins in all living organisms, SO2 is a common air pollutant resulting from the burning of any once-living material such as paper, wood, coal, or petroleum. Petroleum refineries remove much of the Sulfur during the production of gasoline and other products. However, depending on the extent of purification, small amounts of SO2 may still be released during the burning of those products. When SO2 dissolves in water such as in one's lungs or during a rain shower, it also reacts with the H2O forming the weak acid H2SO3. Left in the air, SO2 can be further oxidized to SO3 which dissolves in water to form the much stronger acid, H2SO4. These are part of the cause for acid rain and subsequence acidity of streams and lakes injuring or preventing life in those bodies of water.
Besides the synthesis of Ozone by air pollutants and sunlight, it can also be produced by electrical sparks. It is produced naturally by lightning. But Ozone can also be produced by this means for immediate desirable uses. Ozone is more corrosive that regular Oxygen as a strong oxidizing agent (it steals electrons from other materials) and therefore very toxic to life. As a result it can be used as a very effective disinfectant for killing micro-organisms on food stuffs, in waste water, and for swimming pools. It is also used as a bleach in paper manufacture. Its advantage is, because it is unstable, it disintegrates to regular oxygen leaving no undesirable pollutants.
![]()
to next investigation
to Environmental Chemistry menu
to ie-Chemistry menu
to site menu