![]()
In Biochemistry 4 it was noted that, unlike in metals where rapid electrical current results from the nudging of mobile valence electrons, living nerves transmit electrical signals by a different mechanism. Nerves use a relatively slow avalanche of reactions in large molecules embedded in their cell membranes triggered by flow of ions and smaller molecules. The process begins when sensor proteins, called receptors, in the cell membrane of nerve ends respond to a particular stimuli.
The membranes of nerve cells, like those of all cells, are made of thin (~4 nm) double layers of oily lipids. One end of each lipid molecule is a carboxyl functional group (-COOH colored blue-green in diagrams). Due to uneven distribution of electrical charge caused by Oxygen's attraction for additional electrons, each carboxyl has polar (electrical) attraction to nearby polar water molecules, either inside and outside the cell. The remaining majority of the lipid molecule is a long, flexible, electrically bland chain of 12 to 18 Carbon atoms surrounded by equally bland Hydrogen atoms (-CH2CH2CH2CH2-).  Due to their lack of polarity, these stringy chains, shunned by water, are left to huddle together in the middle of the double layer (colored orange in diagrams ←left and below↓. Shown at left is the structural formula for oleic acid, a common lipid. An invisible Carbon atom lies at the end of each bond which lacks a letter. Each Carbon requires 4 bonds. But for further simplicity, we seldom draw the numerous Hydrogen and their bonds which satisfy that requirement.) As a result of the combination of non-polar chains with contrasting polar ends, these lipid molecules form sheets of skin-like membrane which serve as the packaging of cells.
Due to their lack of polarity, these stringy chains, shunned by water, are left to huddle together in the middle of the double layer (colored orange in diagrams ←left and below↓. Shown at left is the structural formula for oleic acid, a common lipid. An invisible Carbon atom lies at the end of each bond which lacks a letter. Each Carbon requires 4 bonds. But for further simplicity, we seldom draw the numerous Hydrogen and their bonds which satisfy that requirement.) As a result of the combination of non-polar chains with contrasting polar ends, these lipid molecules form sheets of skin-like membrane which serve as the packaging of cells.
Embedded in this sheet of lipid membrane are many proteins (colored Lavender in diagrams), some containing gated pores across the membrane providing passage for otherwise blocked substances. There are a variety of gates. Some open when triggered by physical stimuli such as pressure, light, heat, or electrical potential while others are triggered by chemicals, either charged ions or small electrically neutral molecules. When triggered these gates open allowing passive flow for selected ions or molecules from higher concentration to lower concentration through the cell's membrane.  When ions of only one electric charge are allowed to stream through, a pronounced change in concentration inside the cell can bring sharp change in the electrical potential (measured in Volts) between the interior of a nerve cell and its surrounding environment. This spike in Voltage (like the spiked EKG graph from heart nerves shown above left) typically causes nearby channel gates governed by Voltage to open, amplifying the flood of ions and the electrical signal. The resulting chemical avalanche spreads like a wave across the nerve's outer membrane with the opening of additional gates further away. This actively carries the change in electrical potential along the long nerve axon, transmitting the message.
When ions of only one electric charge are allowed to stream through, a pronounced change in concentration inside the cell can bring sharp change in the electrical potential (measured in Volts) between the interior of a nerve cell and its surrounding environment. This spike in Voltage (like the spiked EKG graph from heart nerves shown above left) typically causes nearby channel gates governed by Voltage to open, amplifying the flood of ions and the electrical signal. The resulting chemical avalanche spreads like a wave across the nerve's outer membrane with the opening of additional gates further away. This actively carries the change in electrical potential along the long nerve axon, transmitting the message.
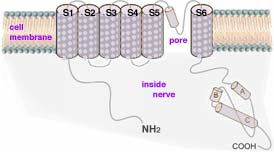 Biochemistry 3 described how odor receptor proteins in cell membranes at nerve endings in the nose catalyze large numbers of adenosine triphosphate (ATP molecules) to lose phosphate groups becoming adenosine monophosphate (cyclic AMP). The cyclic AMP trigger nearby protein gates to snap open allowing a stream of ions though. Each ion gate actually consists of a cluster of four distinct stringy protein, each coiled and twisted into functional shapes. (These channels with their gates are described in Biochemistry 4). The diagram at right → shows one of the 4 subunit proteins artificially stretched out to show that it consists of six coiled segments, each of which crosses the cell membrane. These are designated S1 to S6. Note a seventh, largely uncoiled segment of roughly 20 to 30 amino acids located between S5 and S6 which helps form the pore region and its gate. Normally these seven segments cluster in a tight clump, forming one of four sides of a narrow channel through the membrane (as shown below left). Such channels have been identified in numerous types of cells including nerves, sperm, liver and heart. Gated protein channels are common to nearly all animal cells. But they play a special role in carrying messages along long nerve pathways.
Biochemistry 3 described how odor receptor proteins in cell membranes at nerve endings in the nose catalyze large numbers of adenosine triphosphate (ATP molecules) to lose phosphate groups becoming adenosine monophosphate (cyclic AMP). The cyclic AMP trigger nearby protein gates to snap open allowing a stream of ions though. Each ion gate actually consists of a cluster of four distinct stringy protein, each coiled and twisted into functional shapes. (These channels with their gates are described in Biochemistry 4). The diagram at right → shows one of the 4 subunit proteins artificially stretched out to show that it consists of six coiled segments, each of which crosses the cell membrane. These are designated S1 to S6. Note a seventh, largely uncoiled segment of roughly 20 to 30 amino acids located between S5 and S6 which helps form the pore region and its gate. Normally these seven segments cluster in a tight clump, forming one of four sides of a narrow channel through the membrane (as shown below left). Such channels have been identified in numerous types of cells including nerves, sperm, liver and heart. Gated protein channels are common to nearly all animal cells. But they play a special role in carrying messages along long nerve pathways.
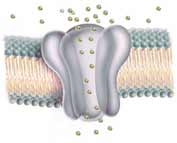
Generally when a receptor protein is triggered by its stimuli (odor, light, pressure, etc.), it causes the formation of cyclic AMP or similar cyclic GDP. Embedded nearby in the same outer membrane of the nerve ending are the ion channels which open when the cyclic AMP or cyclic GDP chemically bind onto each subunit. These cyclic nucleotides attach to the strings of protein which dangle below the pore-forming part of the channel (as shown above). The opening of the channel requires the combined activation of all four subunits. When cyclic nucleotides attach to only two of the proteins composing the walls of the channel, only a miniscule ion flow, about 1% of the maximum, occurs. Attaching three cyclic nucleotides results in about 33% of maximum ion flow compared to that achieved when cyclic nucleotides attach to all four proteins, opening the membrane pore.
Cyclic nucleotide-gated (CNG) ion channels which initiate electrical signals share much of the same structure and function with Voltage-gates channels which respond to the reversal of the normally small electrical potential (measured as negative Voltage relative to the exterior environment). These Voltage-gated channels amplify and carry on that electrical signal along the nerve length. There are an assortment of Voltage-activated channels, each specialized for the passage of K+, Na+, or Ca2+ ions.
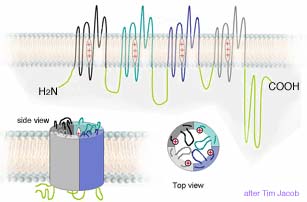 Like the cyclic nucleotide-gated protein channels which initiate the electrical signal, Voltage-gated channels are composed of four repeating pore proteins plus additional proteins inside the nerve. Each pore protein has 6 coiled sections which cross the cell membrane, a pore gate section after the 5th crossing, and a positively charged region on the 4th crossing which acts as a Voltage sensor which in some channels can open the pore in response to a change in electrical potential. The S4 segment of all Voltage-activated channels shares considerable similarity to that of the CNG channels (shown in section above). This S4 segment bears a number of positively charged amino acids at every third position, interspersed by amino acids with hydrophobic (water hating) side groups. In Voltage-gated channels the S4 segment moves when the Voltage is reversed (made positive) opening the channel. Despite their strong similarity, channels triggered by cyclic nucleotides display little Voltage triggered pore activation. So their Voltage sensor segments may be vestiges of their evolutionary orign.
Like the cyclic nucleotide-gated protein channels which initiate the electrical signal, Voltage-gated channels are composed of four repeating pore proteins plus additional proteins inside the nerve. Each pore protein has 6 coiled sections which cross the cell membrane, a pore gate section after the 5th crossing, and a positively charged region on the 4th crossing which acts as a Voltage sensor which in some channels can open the pore in response to a change in electrical potential. The S4 segment of all Voltage-activated channels shares considerable similarity to that of the CNG channels (shown in section above). This S4 segment bears a number of positively charged amino acids at every third position, interspersed by amino acids with hydrophobic (water hating) side groups. In Voltage-gated channels the S4 segment moves when the Voltage is reversed (made positive) opening the channel. Despite their strong similarity, channels triggered by cyclic nucleotides display little Voltage triggered pore activation. So their Voltage sensor segments may be vestiges of their evolutionary orign.
The Voltage gated channels are the biological equivalent of transistors, providing a rapidly changing switch for electric current. In electronic circuitry, a small change in Voltage supplied by a third wire, switches on a much larger electric current flowing in and out of the transistor through the other two wires. In nerves the change in electrical potential acts like a rapid switch to open the Voltage-gates channel resulting in a large flow of ions accompanied by magnified Voltage change. Curiously, the Voltage-gates channels are greater amplifiers that the best man-made commercial transistors.
It might here be appropriate to note that many of the toxins used by poisonous insects to venomous snakes chemically function by reacting with membrane channels in their victims, blocking their vital functions. This gives clue to both the critical importance of these channels and their ubiquitous presence among living organisms. Such poisons have provided a useful tool for distinguishing and studying the various kinds of membrane channel proteins.
One type of gated channel is a cluster of proteins which selectively allow the passage of Calcium ions, Ca2+, driven by a concentration gradient. Generally the Ca2+ concentration inside a cell is much lower (10-7 M) than the Ca2+ concentration immediately outside the cell (~10-3 M). When a receptor is activated as described in Touch & Hearing, Tasting & Smelling and Pain cyclic nucleotides are produced which trigger the gates on Calcium channels to open allowing Calcium ions to stream through from where they are in high concentration to inside the cell where concentration is lower. The resulting temporary increase in Ca2+ inside the cell acts as a second messenger by activating other cellular processes. When these ions migrate to different channel proteins nearby, they trigger those gates to open allowing Potassium ions concentrated inside the nerve to flow outward. This changes electrical potential in the immediate vicinity to the channel which in turn causes still other nearby channels with Voltage-regulated gates to also open allowing additional flow of Calcium, Sodium, and Potassium ions. This in turn causes the opening of other channels a bit further away from the originating detector channel. The opening of the first channels causes a cascade of channels along cell membrane of the nerve to opening until the electrical discharge reaches the far end of the nerve, in some cases as far as 2 meters away.
A number of variations of Calcium channels have been identified by their opening speed, opening duration, electrical responses, molecules which poison their action, and kind of cell where they are found. Calcium channels are not just involved in carrying nerve signals, but also in many cellular functions:
Potassium channels are the most common type of ion channel through cell membranes. Potassium channels are found in most cells, and control the electrical potential (measured as Voltage) of the cell membrane. In neurons, they set the resting membrane potential and determine Voltage response characteristics when pores are triggered to briefly open.

Potassium channels had the unusual ability to allow extremely rapid passage of positively charged Potassium ions, K+, while blocking the flow of chemically very similar but 20% smaller Sodium ions, Na+. This strange feat gained the curiosity of Rod MacKinnon (← photo at left, b 1956) who studied Potassium channels using scorpion venom which by blocking the channel providing a means to study mutated channel proteins. MacKinnon eventually becoming a self-taught x-ray crystallographer to understand the molecular structure and discover many of the features shared by both other channel proteins and utilized by organisms from bacteria to humans. He was awarded half the 2003 Nobel Prize for Chemistry for his achievement.
Because of their electrical charge, ions are electrically attracted to opposite charges. Paradoxically, both (negative) anions and (positive) ca+ions dissolve in water. This is because the water, H/Ö\H, (which is polar due to its uneven charge distribution) can easily accommodate either charge of ion by rotating either to provide its more negative Oxygen or more positive Hydrogen side. Water molecules surround each ion, regardless of its charge, like an entourage surrounds a famous person. This disperses the effective charge partially over the accompanying polarized water, and thus lowers the ion's energy state. (Lower energy states are always preferred; e.g., a ball rolls downhill spontaneously, but never uphill.) 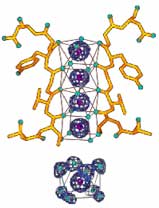 So a Potassium ion, K+ (appearing at bottom of the diagram→ like a purple volleyball), prefers to be surrounded by 8 water molecules rather than to be isolated, to be surrounded by bland non-polar molecules (such as would be required for an unassisted crossing of the lipid cell membrane), or even to remain in most ionic crystal lattices. (The low energy state of the hydrated ions is why even the tightly bonded ionic salts such as KCl and NaCl dissolve in water without needing addition of energies of fusion and vaporization.) But the crystallography evidence suggests that the protein channel through the cell membrane is too narrow to allow ions to pass through accompanied by their water entourage. MacKinnon found that the dilemma was solved by amino acid side groups lining the walls of the protein channel. They are ideally spaced so that the channel provides a series of four twisted-cube-shaped pockets with carbonyl functional groups (C/Ö\H, highlighted in the diagram with colored Oxygen) on the corners capable of acting as surrogate water. This allows K+ ions to abandon their water just inside the nerve cell's outer membrane, then jump from pocket to pocket maintaining the same low energy state until them to regain new water entourages just outside the nerve. While the diagram shows Potassium ions in all four pocket positions, actually only two K+ pass through the narrow passage at at any instant in time, with water in the alternating pockets.
So a Potassium ion, K+ (appearing at bottom of the diagram→ like a purple volleyball), prefers to be surrounded by 8 water molecules rather than to be isolated, to be surrounded by bland non-polar molecules (such as would be required for an unassisted crossing of the lipid cell membrane), or even to remain in most ionic crystal lattices. (The low energy state of the hydrated ions is why even the tightly bonded ionic salts such as KCl and NaCl dissolve in water without needing addition of energies of fusion and vaporization.) But the crystallography evidence suggests that the protein channel through the cell membrane is too narrow to allow ions to pass through accompanied by their water entourage. MacKinnon found that the dilemma was solved by amino acid side groups lining the walls of the protein channel. They are ideally spaced so that the channel provides a series of four twisted-cube-shaped pockets with carbonyl functional groups (C/Ö\H, highlighted in the diagram with colored Oxygen) on the corners capable of acting as surrogate water. This allows K+ ions to abandon their water just inside the nerve cell's outer membrane, then jump from pocket to pocket maintaining the same low energy state until them to regain new water entourages just outside the nerve. While the diagram shows Potassium ions in all four pocket positions, actually only two K+ pass through the narrow passage at at any instant in time, with water in the alternating pockets.
The smaller Sodium ions, Na+, could not be simultaneously close to all corner carbonyl groups, so they are unable to achieve inside the pockets the low energy state essential for shedding their water entourage. As a result the larger Potassium ions flood through the open channel at a rate of 107 to 108 ions/second while the Sodium ions are prohibited by an energy barrier from even entering. This ion selection mechanism works so well that it has apparently been continually used by living cells without significant change since some of the earliest life on earth.
There are over 80 mammalian genes that encode various Potassium channel proteins. Some potassium channels have gates which are triggered to open by Calcium ions, Ca2+, while others are Voltage-gated and open in response to the reversal of the normally negative Voltage inside the cell. Typically the various trigger mechanisms and gate assemblies occupy the inner half of the channel.
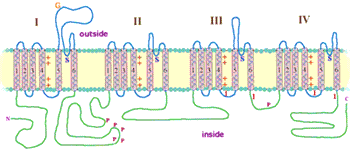
The Sodium channel is formed by four proteins very much like the Calcium and Potassium channels. The outside (extracellular) portion of the pore is formed by the (blue) region between coiled sections 5 and 6 in each of the four channel proteins (labeled I to IV). These sections of the proteins form the narrowest part of the pore and are responsible for its ion selectivity. The section linking proteins III and IV is also important for channel function. This region plugs the channel after prolonged activation. The pore of Sodium channels contains a selection filter made of negatively charged amino acids, which attract the positive Na+ ion and keep out negatively charged ions such as chloride, Cl–. The Na+ cations flow into the constricted part of the pore that is 0.3 by 0.5 nm wide, just large enough to allow a single Na+ ion with an accompanying water molecule to pass through. The larger K+ ion cannot fit through this restricted pore. In addition, ions of different sizes cannot interact as well with the negatively charged glutamic acid which line the pore.
Voltage-gated sodium channels play an key role in the electrical potential spikes. If enough channels open, Na+ ions will move into the cell depolarizing the cell. The more Na+ channels in a neuron's membrane, the faster the electrical potential signal will propagate. Na+ channels both open and close more quickly than K+ channels, producing an influx of positively charged Na+ toward the beginning of the action potential (Voltage spike) and an outflow of K+ toward the end. The ability of these channels to assume a closed-inactivated state causes the refractory period and is critical for the propagation of action potentials along an axon.
 In 1957 Danish chemist Jens Christian Skou (b 1918, photo at right→) published in Biochimica et Biophysica Acta a paper entitled
In 1957 Danish chemist Jens Christian Skou (b 1918, photo at right→) published in Biochimica et Biophysica Acta a paper entitled The Influence of some Cations on an Adenosine Triphosphatase from Peripheral Nerves
in which he described an enzyme (a protein) which uses energy carried by the ATP molecule to actively pumped Sodium ions (Na+) and Potassium ions (K+) across cell membranes. He received half the 1997 Nobel Prize in Chemistry for the first discovery of an ion-transporting enzyme: Na+, K+ –ATPase. (The standard format used for naming an enzyme is to add the suffix -ase to a description of the catalyzed chemical reaction.)
It is not clear why early life on earth evolved such an ion sorting pump. Perhaps is was one of a variety of early mechanisms used to store the energy necessary to maintain life. But cells have evolved to require a low concentration of Sodium ions and high concentration of Potassium ions within the cell compared to the environment outside the cells. Due to a difference in their attraction for valence electrons as well as a somewhat greater ability of Potassium ions to cross the cell membrane, this segregation of ions produces a small (~ -70 mV) electric potential occurs with the cell interior negative compared to the external environment.
This resting
electrical potential forms the basis for both the actions of nerves and muscles. In addition, export of Sodium from the cell provides the driving force for several other mechanisms which import glucose, amino acids and other nutrients into the cell. And movement of Sodium from one side of an epithelium to the other side creates an osmotic gradient that drives the absorption of water. Another important task of the Na+-K+ pump is to provide a Na+ gradient that is used by certain carrier processes. In the gut, for example, Sodium is transported out of the resorbing cell on the blood side via the Na+-K+ pump, whereas, on the resorbing side, the Na+-Glucose transporter uses the created Na+ gradient as a source of energy to import both Na+ and Glucose, which is far more efficient than simple diffusion. Similar processes are located in the kidney system.
1. Three Sodium ions (Na+), normally surrounded by water of hydration, are attracted into a cavity inside the protein embedded in the cell membrane by carbonyl functional groups lining the cavity. The attraction to the carbonyl groups must be stronger than that to the hydration water which they leave behind.
2. Following the bonding of the three Na+, a section of the protein dangling inside the cell attracts and reactions with an ATP molecule, resulting in one of the phosphates bonding to the protein, bringing with it much of the energy stored previously in its bond to ATP. The influence of the phosphate's oxygen and energy causes the cavity to twist, narrowing the channel's inner neck while widening the outer passage.
3. The change in protein shape perhaps enlarges the cavity reducing the ability of functional groups lining the cavity to attract the Na+. The ions, now finding stronger attraction to water in the exterior environment, depart from the cavity leaving it empty.
4. The cavity is now attractive to two, 25% larger Potassium ions, K+, to abandon surrounding water of hydration by attraction to the carbonyl functional groups lining the protein cavity.
5. The presence of the K+ bonded to the protein's functional groups is apparently sufficiently different from the situation with attached Na+ to result in the phosphate group being released which results in the outer cavity opening closing and the inner one reopening.
6. Much as the change in cavity shape encouraged the earlier release of Na+, the now probably smaller cavity size when open inward may crowd the K+ enough to encourage them to abandon attraction to the carbonyl groups in exchange for being surrounding by water inside the cell. With the cavity again empty, the protein pump is ready to repeat the process of pumping Sodium out and Potassium into the cell.
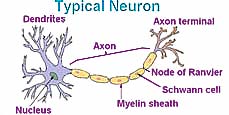 In the long, thread-like axon portion of a nerve, specialized Schwann cells typically provide a covering of water insoluble, fatty myelin sheath with a high dielectric constant, which insulates the voltage spike (action potential) from the fading effect of ions. This allows the spike in electrical potential to move more rapidly along the axon (about 100 m/sec compared to less than 5 m/sec were membranes lack myelin). The signal is, re-amplified by additional gated channels located at breaks in the myelin sheath called nodes of Ranvier. Degeneration of the myelin sheath results in the fading of nerve impulses creating a disease known as multiple sclerosis causing various symptoms depending upon which signals are interrupted. The name multiple sclerosis (MS) refers to the multiple scars (or scleroses) on the myelin sheaths. It appears that multiple sclerosis results from a mistaken attack of a person's immune system upon the myelin sheath or the cells which make and maintain it, and thus it is considered an autoimmune disease.
In the long, thread-like axon portion of a nerve, specialized Schwann cells typically provide a covering of water insoluble, fatty myelin sheath with a high dielectric constant, which insulates the voltage spike (action potential) from the fading effect of ions. This allows the spike in electrical potential to move more rapidly along the axon (about 100 m/sec compared to less than 5 m/sec were membranes lack myelin). The signal is, re-amplified by additional gated channels located at breaks in the myelin sheath called nodes of Ranvier. Degeneration of the myelin sheath results in the fading of nerve impulses creating a disease known as multiple sclerosis causing various symptoms depending upon which signals are interrupted. The name multiple sclerosis (MS) refers to the multiple scars (or scleroses) on the myelin sheaths. It appears that multiple sclerosis results from a mistaken attack of a person's immune system upon the myelin sheath or the cells which make and maintain it, and thus it is considered an autoimmune disease.
The mechanism of nerve transmission: When a stimulus such as described in Touch & Hearing, Tasting & Smelling and Pain is detected by a receptor G-protein embedded in a nerve, many ATP molecules are catalytically decomposed to cyclic AMP, some of which trigger Calcium channels to open allowing Calcium ions more abundant outside the nerve to flow into the nerve cell. These Ca2+ trigger nearby Potassium channels to open allowing millions of Potassium ions concentrated inside the nerve to rapidly leak out of the cell, reversing the electrical potential across the cell membrane. The reversal of electrical potential across the nerve membrane is amplified by triggering additional Voltage-gated Potassium channels and Sodium channels to open, drastically decreasing K ion concentration inside the membrane while increasing the Na+ inside. The sharp spike in electrical potential passes along the long narrow nerve axon until this wave of ion motion, occasionally re-amplified by Voltage-gated channels, reaches the far terminal end of the nerve. A cyclic mechanism then uses ATP to pump Potassium into the cell and Sodium out, restoring the resting electrical potential of the nerve.
There is an old idea that our nervous system can be swamped with information. That we can increasingly tolerate the shower temperature being gradually increased to warmer temperatures, that spicy food becomes easier to eat as we eat more of them, that after a while of listening loud music doesn't seem so loud, that a tooth ache or injury becomes more tolerable with time. Investigating this phenomena could be dangerous, so carefully consider and adopt safety precautions before starting and re-evaluate them periodically during your investigation. You might wish to investigate your sensitivity to a taste or an odor as it might change with exposure over time. Try to create a way to make your investigation quantifiable. Could this be done using standards for comparison? Is there any way to tell if your detection of the standard varies? Is the minimum detectable concentration constant?
Communicating technical information such as observations and findings is a skill used by scientists but useful for most others. If you need course credit, use your observations in your journal to construct a formal report.
![]()
to next investigation
to Biochemistry menu
to ie-Chemistry menu
to site menu