![]()
(You don't really know where you are unless you know how you got here.)
![]()
 The ancient Greeks such as Galen of Pergamum (b129AD, d~200AD) tried to understand anatomy and how a living body functions. Galen's writings were taught to succeeding generations with some of his views still a part of common culture today. Following the Dark Ages, those centuries when much of Greek understanding was ignored by European civilization, the Renaissance attempted to revive the best of ancient culture. Less than a century after Gutenberg's printing technology heralded a proliferation of books, Andrea Vesalius (←woodcut at left, b1514 ,d1564) studied medicine and Galen's anatomy in Belgium and France, finally receiving a doctorate at Padua, Italy, in 1537. After writing several preliminary works, Vesalius published in 1543 De Humanis Corporis Fabrica contrasting Galen's description of assumed human anatomy with facing page drawings of dissected human cadavers. Numerous discrepancies between text and diagrams were obvious. While it remained illegal to desecrate even the bodies of executed criminals, Vesalius' book based on such dissections revolutionize the understanding of human anatomy and the functions of its organs.
The ancient Greeks such as Galen of Pergamum (b129AD, d~200AD) tried to understand anatomy and how a living body functions. Galen's writings were taught to succeeding generations with some of his views still a part of common culture today. Following the Dark Ages, those centuries when much of Greek understanding was ignored by European civilization, the Renaissance attempted to revive the best of ancient culture. Less than a century after Gutenberg's printing technology heralded a proliferation of books, Andrea Vesalius (←woodcut at left, b1514 ,d1564) studied medicine and Galen's anatomy in Belgium and France, finally receiving a doctorate at Padua, Italy, in 1537. After writing several preliminary works, Vesalius published in 1543 De Humanis Corporis Fabrica contrasting Galen's description of assumed human anatomy with facing page drawings of dissected human cadavers. Numerous discrepancies between text and diagrams were obvious. While it remained illegal to desecrate even the bodies of executed criminals, Vesalius' book based on such dissections revolutionize the understanding of human anatomy and the functions of its organs.
 Two centuries later, European royalty often found it entertaining to be shocked by new machines, which when rotated, generated electric sparks. Luigi Galvani (b1737, d1798, portrait at right→), professor of Anatomy at the University of Bologna, investigated contractions in dissected frog legs caused by such electrical sparks. Later he investigated a possible connection between what he called this animal electricity and proposals from people such as Benjamin Franklin that lightning storms might also involve electricity. To investigate, Galvani suspended frog legs by metal hooks outdoors on a clothes line in a lightning storm. Disappointed after a day with no observed effects, Galvani returned the frog legs to hanging in a storage cabinet. With the metal hooks draped from the metal of the cabinet, the large frog muscles quickly started twitching!
Two centuries later, European royalty often found it entertaining to be shocked by new machines, which when rotated, generated electric sparks. Luigi Galvani (b1737, d1798, portrait at right→), professor of Anatomy at the University of Bologna, investigated contractions in dissected frog legs caused by such electrical sparks. Later he investigated a possible connection between what he called this animal electricity and proposals from people such as Benjamin Franklin that lightning storms might also involve electricity. To investigate, Galvani suspended frog legs by metal hooks outdoors on a clothes line in a lightning storm. Disappointed after a day with no observed effects, Galvani returned the frog legs to hanging in a storage cabinet. With the metal hooks draped from the metal of the cabinet, the large frog muscles quickly started twitching!
 After reading Galvani's 1791 reported findings, Alessandro Volta of Como, Italy (←portrait at left with apparatus, b1745 ,d1827) suggested the twitches might have been caused by electricity generated between the metal of the hooks and a dissimilar metal of the cabinet. In 1800 he assembled a sandwich of two dissimilar metals attempting to test this hypothesis. But he was unsure whether there was a tiny electrical effect or none. So he tried to amplify which might be a small effect by stacking multiple layers of such metal sandwiches, interspersed by brine (NaClaq) soaked cardboard. Volta found that when he brought a wire attached to the bottom metal near the top metal, a spark occurred. That discovery provided the first reliable device to generate a continuous flow of electric current. Such a Voltaic pile eventually became known as a battery. (Historically the term battery referred to a group of ordnance such as cannon coordinated to optimize their destructive power. Benjamin Franklin first applied the term battery to the amplified electrical power of parallel Leyden jars storing electric charge from one of the common spark generators. Eventually the term came to mean a group of multiple Voltaic cells. Nearly any two dissimilar metals will work!) The ability to generate continuous electric current rapidly led to investigations and understanding of many electric phenomena and eventually to today's electronic age.
After reading Galvani's 1791 reported findings, Alessandro Volta of Como, Italy (←portrait at left with apparatus, b1745 ,d1827) suggested the twitches might have been caused by electricity generated between the metal of the hooks and a dissimilar metal of the cabinet. In 1800 he assembled a sandwich of two dissimilar metals attempting to test this hypothesis. But he was unsure whether there was a tiny electrical effect or none. So he tried to amplify which might be a small effect by stacking multiple layers of such metal sandwiches, interspersed by brine (NaClaq) soaked cardboard. Volta found that when he brought a wire attached to the bottom metal near the top metal, a spark occurred. That discovery provided the first reliable device to generate a continuous flow of electric current. Such a Voltaic pile eventually became known as a battery. (Historically the term battery referred to a group of ordnance such as cannon coordinated to optimize their destructive power. Benjamin Franklin first applied the term battery to the amplified electrical power of parallel Leyden jars storing electric charge from one of the common spark generators. Eventually the term came to mean a group of multiple Voltaic cells. Nearly any two dissimilar metals will work!) The ability to generate continuous electric current rapidly led to investigations and understanding of many electric phenomena and eventually to today's electronic age.
We now understand that each kind of atom has its own characteristic strength of attraction for its outer constituents called electrons. Atoms in metals (the subgroup of the chemical elements which readily conduct electricity) only weakly attract their outer-most electrons. Within a piece of metal composed of a large number of identical atoms, these outer valence electrons can move with little resistance as long as they remain near the metal atoms. If a few extra electrons are supplied to one point on a metallic object, the freely flowing sea-like valence electrons can move slightly (adjusting at the speed of light within the metal) so that the excess can be drawn off at some distant location nearly instantaneously. The spark from Volta's electric battery is caused by each metal having a different amount of attraction for its valence electrons, something we measure as electrical potential in units appropriately named Volts. When two different metals compete for electrons available from chemical reactions with the brine, the metal with the stronger attraction for additional electrons pulls some electrons away from the other metal. The lack of direct contact between the two metals forces any actual exchange of electrons to flow through the external connecting wire. Along the way, that flow of electric current can generate heat, light, or do other work (such as causing a twitch or carry a message such as pain, our reason for considering all this).
The animal electricity investigated by Galvani flows along the outer membrane a particular kind of long, wire-shaped cell commonly called a nerve, or more formally a neuron. But unlike electric current flowing through metallic wires, the transmission speed is slower by more than 10-6, and does not involved the flow of weakly held electrons. Instead the transmission of the electrical signal along a nerve involves much slower chemical reactions and floods of ions and molecules. The process of animal electricity can be divided into at least four distinct functions:The early electricians who experimented with electricity would often shock themselves to measure the power of new electrical devices, much as royalty found it entertaining to be shocked by new spark generating machines. Right from the beginning there seemed to be a connection between the pain of the electric shock and a pleasure it sometimes seemed to bring. (When later machines became powerful enough to cause death by electrocution, electricians became more cautious.) The connection between pleasure and pain still remains somewhat elusive (perhaps because the connection may not be universal or innate).
In general pain is the mechanism a body uses to signal that something is either wrong or in danger of injury or other system failure. To most women who have given childbirth or anyone having experienced the tubal pain caused by appendicitis, a kidney stone or a gallstone, there seems to be little in common between those shrill pains and pleasure provided by other stimuli. Unlike the sense of pleasure which seems to be largely learned by associations with other pleasures, the ability to detect pain appears to be innate, developing very early accompanying the physical development of the nervous system. (That pleasure is likely learned may help account for how mildly painful stimuli might also provide pleasure. Because pleasure seems to be a more complex, its consideration will be postponed until Biochemistry 10: Pleasure). We will consider below how pain is detected, how the process works, and how pain compares with other sensory experiences. This will require a closer look at the construction and operation of the protein gates which control passage of ions and molecules into and out of cells such as neurons.
All information which we receive comes to us via long, stringy, self folding and coiling proteins which reside in the outer surface membranes of certain cells. These receptor molecules have relatively small variations which allow them to detect various aspects of their environments.
Most detection of environmental stimuli involve variations of G-proteins which get their name from being supplied energy by guanosine triphosphate, GTP. (G-proteins and their sensory mechanism were described in detail in the previous Biochemistry 3 which also described the basic chemistry of proteins.) G-proteins are widely used by many body systems to detect particular molecules and ions. Besides some being specialized to detect particular odors and tastes, others detect hormones such as adrenaline. G-proteins have a wide diversity of function in many organisms. For example in primitive yeast the G-proteins are used to detect and chose a mate! G-proteins are members of a still larger super-family of proteins which thread 7 times back and forth across the cell membrane.
A closely related group of proteins are similar but have only 6 membrane crossings. In place of the crossing section between the fifth and last crossings (where the 6th crossing is missing) are 20 to 30 amino acids which help form a gated pore through the cell membrane which control the passage of ions and larger molecules. Each pore or channel through the membrane is surrounded by four such channel-proteins, each contributing a quarter of the lining of the pore wall. To fully open a single pore requires four cyclic-AMP molecules, each activating one of the channel-proteins. When one or two cyclic-AMP nucleotides attach, the channel only opens slightly (~1% of the maximum ion flood current). The channel opens more when a third nucleotide attaches (~33%), and opens fully when the fourth becomes chemically bonded. The segment of each channel-protein which attaches to the cyclic-AMP hangs below the pore-forming region inside the cell, much like the location where GDP attaches to the odor detector G-protein. (See details in the previous investigation). Such pore openings are not exclusive to nerve receptors, but similar channels have been identified in the light detecting rod and cone cells in eyes, the flagellum of mammal sperm, and within brain, liver and heart tissue.
The fourth crossing segment of all these proteins show an additional similarity to still another family of Voltage gated channel proteins which are activated by changes in electrical potential and also function to magnify the strengths of electrical potential spikes which carry nerve signals.
 Cell membranes are composed of lipids. These molecules have long oily, electrically-bland, non-polar tails [colored tan in the ←structural formula to left and diagrams below and elsewhere in these Biochemistry pages]. As a result this portion of the lipids have low solubility in water. Each lipid also has an electrically-polar, water attracting carboxyl group on one end [colored blue-green in the diagrams]. These lipid molecules align in a double layer, forming membrane surfaces such as a cell's outer cell membrane and other membranes inside he cell. Their water-loving carboxyl groups are attracted towards the aqueous solutions which are prevalent both inside and outside most cells. Generally the long, non-polar tails are flexible except where a double bond forces a stiff kink. As a result the tails from both layers intertwine creating the flexible strength suitable for life's packaging material.
Cell membranes are composed of lipids. These molecules have long oily, electrically-bland, non-polar tails [colored tan in the ←structural formula to left and diagrams below and elsewhere in these Biochemistry pages]. As a result this portion of the lipids have low solubility in water. Each lipid also has an electrically-polar, water attracting carboxyl group on one end [colored blue-green in the diagrams]. These lipid molecules align in a double layer, forming membrane surfaces such as a cell's outer cell membrane and other membranes inside he cell. Their water-loving carboxyl groups are attracted towards the aqueous solutions which are prevalent both inside and outside most cells. Generally the long, non-polar tails are flexible except where a double bond forces a stiff kink. As a result the tails from both layers intertwine creating the flexible strength suitable for life's packaging material.

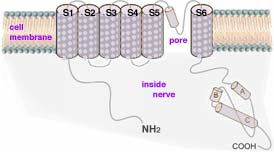 Charles Scott Sherrington (←photo left, 1932 Nobel Prize in Physiology/Medicine, b1857, d1952) discovered in 1906 specialized nerve receptors, now called nociceptors, responsible for initiating pain. Nociceptors develop from neural crest stem cells. Differentiation occurs and two different types of nociceptors are formed expressing distinct repertoires of ion channels and sensors. The molecular sensors in these cells are activated by harmful chemical or physical conditions. The first identified pain sensor, found imbedded in the outer surface membrane of a nociceptor, is TRPV1 (transient receptor potential cation channel, subfamily V, member #1), a protein string of 838 amino acids and molecular mass of 95 kDa. This pain detector protein contains BOTH sensor and gated pore sections. TRPV1 is thus a member of the family of proteins (depicted in diagram above right→) which have coiled sections which thread across the cell membrane 6 times. In the part of the long protein where G-proteins have their sixth of seven coiled sections crossing the cell membrane, this protein has a sequence of amino acids which, when triggered by the sensor, changes shape serving as a selective gateway. This gated channel, when open, selectively allows electrically charged ions to pass through the outer membrane of the nerve cell.
Charles Scott Sherrington (←photo left, 1932 Nobel Prize in Physiology/Medicine, b1857, d1952) discovered in 1906 specialized nerve receptors, now called nociceptors, responsible for initiating pain. Nociceptors develop from neural crest stem cells. Differentiation occurs and two different types of nociceptors are formed expressing distinct repertoires of ion channels and sensors. The molecular sensors in these cells are activated by harmful chemical or physical conditions. The first identified pain sensor, found imbedded in the outer surface membrane of a nociceptor, is TRPV1 (transient receptor potential cation channel, subfamily V, member #1), a protein string of 838 amino acids and molecular mass of 95 kDa. This pain detector protein contains BOTH sensor and gated pore sections. TRPV1 is thus a member of the family of proteins (depicted in diagram above right→) which have coiled sections which thread across the cell membrane 6 times. In the part of the long protein where G-proteins have their sixth of seven coiled sections crossing the cell membrane, this protein has a sequence of amino acids which, when triggered by the sensor, changes shape serving as a selective gateway. This gated channel, when open, selectively allows electrically charged ions to pass through the outer membrane of the nerve cell.
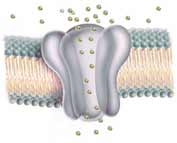
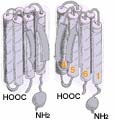 The flattened 2-dimensional diagram (above ↑) shows the various sections of one channel protein. In an actual cell membrane the coiled crossing sections clump together (←as in the 3-dimensional diagram at left showing two of the four channel proteins). Generally ion channels through cell membranes require four proteins to form the gated passageway. (←Here the front half of the cluster is not shown; these two proteins form the back half of the channel.) The more flexible, only partially coiled 20 to 30 amino acids between the 5th and the last crossing of each protein provide the gate function which blocks or permits passage of ions. The semi-transparent diagram below left (↵) provides a still less detailed image of how four TRPV1 proteins form the walls of the central channel through the cell membrane.
The flattened 2-dimensional diagram (above ↑) shows the various sections of one channel protein. In an actual cell membrane the coiled crossing sections clump together (←as in the 3-dimensional diagram at left showing two of the four channel proteins). Generally ion channels through cell membranes require four proteins to form the gated passageway. (←Here the front half of the cluster is not shown; these two proteins form the back half of the channel.) The more flexible, only partially coiled 20 to 30 amino acids between the 5th and the last crossing of each protein provide the gate function which blocks or permits passage of ions. The semi-transparent diagram below left (↵) provides a still less detailed image of how four TRPV1 proteins form the walls of the central channel through the cell membrane.
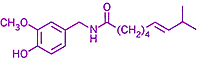 TRPV1 is very sensitive to both heat and to capsaicin (a pungent extract of the Capsicum pepper family, structural formula right→). TRPV1 has a preference for allowing the passage of ions with an electric charge of 2+ (Ca2+ > Mg2+ > Na+ ≈ K+ ≈ Cs+). Ca2+ is especially important to TRPV1 function. Ca2+ originating outside the nerve cell helps to eventually desensitize the nerve, a process which enables the nerve to adapt to the particular chemical or physical cause of the pain, eventually reducing the nerve's response so the pain diminishes.
TRPV1 is very sensitive to both heat and to capsaicin (a pungent extract of the Capsicum pepper family, structural formula right→). TRPV1 has a preference for allowing the passage of ions with an electric charge of 2+ (Ca2+ > Mg2+ > Na+ ≈ K+ ≈ Cs+). Ca2+ is especially important to TRPV1 function. Ca2+ originating outside the nerve cell helps to eventually desensitize the nerve, a process which enables the nerve to adapt to the particular chemical or physical cause of the pain, eventually reducing the nerve's response so the pain diminishes.
 The question remains: How does the channel detect pain? Pain caused by heat may be the easiest to understand. It has been long known that the shape of a protein can be denatured by both heat and other harsh conditions. A protein's critical coiled and folded shape is maintained by weak chemical bonds between various functional groups along the long amino-acid chain. As temperature is increased, increasing vibrational motions can cause the dissociation of these weak bonds, resulting in the protein changing shape. In the case of TRPV1, a rise in temperature can cause enough changes near the pore to cause it to open, allowing ions to flood through setting off the nerve impulse that is subsequently interpreted by the brain as pain. Other mechanical threats such as pulling a hair or a puncture wound likely use those physical motions to trigger open pores, initiating the signal of pain transmitted to the brain.
The question remains: How does the channel detect pain? Pain caused by heat may be the easiest to understand. It has been long known that the shape of a protein can be denatured by both heat and other harsh conditions. A protein's critical coiled and folded shape is maintained by weak chemical bonds between various functional groups along the long amino-acid chain. As temperature is increased, increasing vibrational motions can cause the dissociation of these weak bonds, resulting in the protein changing shape. In the case of TRPV1, a rise in temperature can cause enough changes near the pore to cause it to open, allowing ions to flood through setting off the nerve impulse that is subsequently interpreted by the brain as pain. Other mechanical threats such as pulling a hair or a puncture wound likely use those physical motions to trigger open pores, initiating the signal of pain transmitted to the brain.
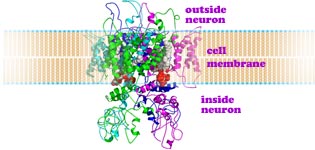 The nociceptor's response to a chemical threat might be understand by recalling the description in Biochemistry 3 of the mechanism for smelling. The attachment of the odor molecule and subsequent reaction with a GTP energy carrying molecule causes a shape change ejecting the odor molecule and setting off a sequence of chemical changes which eventually causes nearby protein channels to open. In the pain mechanism, the shape change directly opens TRPV1's own channel. This immediate route for ion passage probably provides both faster and more sharp response than does odor or taste detection. (At right → is a structural diagram for TRPV1. The four channel proteins are distinguished by different colors. The coiled, globular, and other sections are here depicted in close to their true proportional sizes and locations.)
The nociceptor's response to a chemical threat might be understand by recalling the description in Biochemistry 3 of the mechanism for smelling. The attachment of the odor molecule and subsequent reaction with a GTP energy carrying molecule causes a shape change ejecting the odor molecule and setting off a sequence of chemical changes which eventually causes nearby protein channels to open. In the pain mechanism, the shape change directly opens TRPV1's own channel. This immediate route for ion passage probably provides both faster and more sharp response than does odor or taste detection. (At right → is a structural diagram for TRPV1. The four channel proteins are distinguished by different colors. The coiled, globular, and other sections are here depicted in close to their true proportional sizes and locations.)
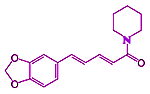 Like many biochemical molecules, TRPV1 is utilized for a variety of functions. Besides being capable of detecting and warning of harmful situations and mediating pain from other receptors, it also is used for tasting foods. Not only are TRPV1 located in our mouths and other part of our body sensitive to capsaicin from the hot chili peppers originating in the Americas, TRPV1 are also sensitive to piperine. The piperine molecule, abundant in pepper formed by the grinding of peppercorns from India and used for food seasoning, was first identified by Hans Christian Ørsted in 1819 (←its now known structural formula is at left).
Like many biochemical molecules, TRPV1 is utilized for a variety of functions. Besides being capable of detecting and warning of harmful situations and mediating pain from other receptors, it also is used for tasting foods. Not only are TRPV1 located in our mouths and other part of our body sensitive to capsaicin from the hot chili peppers originating in the Americas, TRPV1 are also sensitive to piperine. The piperine molecule, abundant in pepper formed by the grinding of peppercorns from India and used for food seasoning, was first identified by Hans Christian Ørsted in 1819 (←its now known structural formula is at left).
The electrical Voltage caused by the changing ion concentration must reach a threshold value before it is strong enough to initiate the series of events which allows for the conscious awareness of pain. (See Biochemistry B5: Nerve Messaging and Biochemistry B6: Contact by Nerve for details about how a a signal is sent along a neuron then transferred to another neuron.) The nociceptors are classified by which of the environmental stimuli they respond, either chemical, thermal, or mechanical. Some nociceptors respond to more than one of these so are designated polymodal. Other nociceptors normally respond to none of these stimuli, although they may respond to stimulation under conditions of inflammation. They are referred to as sleeping or silent nociceptors.
There's been a long debate whether itch and pain sensations are caused by separate nerves, or by the same ones. Perhaps itching as just a less intense version of pain. In 2007 a group led by Zhou-Feng Chen, discovered a receptor that enabled neurons in the spine to communicate itch sensations to the brain. That family of proteins known as Mrg receptors were thought to be the primary sensors of pain signals. To determine their function, Xinzhong Dong, David Anderson, and their colleagues bred mice that lacked a cluster of about half of the Mrg genes. They expected these mice to be less sensitive to pain. But after running a battery of tests, they found that they were at least as sensitive to pain as normal mice. Wondering whether the mysterious Mrg receptors were not pain receptors at all, but rather, itch receptors, they injected two sets of mice with a series of itch-inducing compounds. The first compound tried was chloroquine, simply because it was the cheapest itch-inducing drug. The mice with the Mrg receptors scratched at their itches, but those without the Mrg cluster didn't scratch nearly as much. When they did scratch, it was only after a pronounced delay. Further experiments indicated that this was probably in response to a chloroquine provoked release of histamine in the skin. They knew mice without the Mrg gene could respond normally to itch-inducing histamine. They concluded that chloroquine causes itching primarily by binding to an Mrg receptor on nerve fibers in skin. After evaluating each of the genes, the researchers confirmed that a single gene, MrgA3, accounted for the sensitivity to chloroquine. Sifting through human Mrg genes, they again found only one, MrgX1, that caused chloroquine sensitivity. Dong and his colleagues found that the chloroquine sensitive neurons in mice also respond to a separate itch causing substance known as BAM8-22, apparently via a receptor known as MrgC11. The same neurons respond as well to histamine, via histamine receptors. Their data raise the possibility that there is a small and specific family of neurons which only respond to itch-inducing compounds. These neurons may exclusively create itch sensations. Dong also hopes that his group will clear up another issue. Itch and pain sensations antagonize each other, so that when you feel an itch and you scratch it, the scratch induces pain and suppresses the itch. That interaction is remains poorly understood.
 A tarantula native to the West Indies emits a venom which also activates TRPV1 channels causing searing pain. There is hope that by understanding such molecules which trigger the opening of pain channels in nerve endings, molecules might be found to block the channel. Such a molecule might alleviate the intense pain of burns and the sting due to venomous plants and animals.
A tarantula native to the West Indies emits a venom which also activates TRPV1 channels causing searing pain. There is hope that by understanding such molecules which trigger the opening of pain channels in nerve endings, molecules might be found to block the channel. Such a molecule might alleviate the intense pain of burns and the sting due to venomous plants and animals.
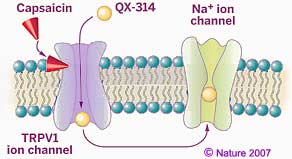 As an example of how such knowledge is applied, an injectable local anesthetic QX-314 (shown at left above) blocks pain without causing the paralysis or numbness caused by earlier local anesthetics. The pain relief is provided by combining capsaicin with QX-314, a variation of lidocaine (the first amino amide-type anesthetic, developed in 1943; this variation has a third -CH2CH3 ethyl group on the positively charged amine tail in the structure as shown above). The combination blocks transmission of pain signals but does not block nerve pulses which control movements or non-painful sensations. The combination works by blocking Sodium ion channels in nerves that sense pain, thereby preventing the nerves from transmitting pain signals to the brain. Earlier forms of local anesthetics which are electrically neutral, pass by osmosis through the non-polar lipid membranes into nerve cells then plug the nerves' Sodium channels from the inside. However such anesthetics also enter all nearby nerve cells and so block all nerve signals including those which carry information unrelated to pain. Because of its positive electric charge, QX-314 is unable to get passively through the lipid membrane of nerve cells by osmosis. But the accompanying capsaicin provides QX-314 entry only to nociceptors by triggering opening of TRPV1 ion channels. After entering only nociceptors through these TRPV1 channels, QX-314 then plugs the numerous Sodium channels, blocking amplification of the Voltage spike needed to carry the pain message along the neuron. Because the TRPV1 ion channels are not found in the cell membranes of other kinds of neurons, such a combination of capsaicin and QX-314 selectively deactivates only pain signals.
As an example of how such knowledge is applied, an injectable local anesthetic QX-314 (shown at left above) blocks pain without causing the paralysis or numbness caused by earlier local anesthetics. The pain relief is provided by combining capsaicin with QX-314, a variation of lidocaine (the first amino amide-type anesthetic, developed in 1943; this variation has a third -CH2CH3 ethyl group on the positively charged amine tail in the structure as shown above). The combination blocks transmission of pain signals but does not block nerve pulses which control movements or non-painful sensations. The combination works by blocking Sodium ion channels in nerves that sense pain, thereby preventing the nerves from transmitting pain signals to the brain. Earlier forms of local anesthetics which are electrically neutral, pass by osmosis through the non-polar lipid membranes into nerve cells then plug the nerves' Sodium channels from the inside. However such anesthetics also enter all nearby nerve cells and so block all nerve signals including those which carry information unrelated to pain. Because of its positive electric charge, QX-314 is unable to get passively through the lipid membrane of nerve cells by osmosis. But the accompanying capsaicin provides QX-314 entry only to nociceptors by triggering opening of TRPV1 ion channels. After entering only nociceptors through these TRPV1 channels, QX-314 then plugs the numerous Sodium channels, blocking amplification of the Voltage spike needed to carry the pain message along the neuron. Because the TRPV1 ion channels are not found in the cell membranes of other kinds of neurons, such a combination of capsaicin and QX-314 selectively deactivates only pain signals.
QX-314 is a local anesthetic. But because some people such as those with burns or arthritis experience prolonged pain, there has been hope of finding a substance which might be used as a medication to block the TRPV1 output signal. However early attempts have found an adverse side effect. While it was understood that temperature above 43°C produce the sensation of burn pain, apparently TRPV1 also helps monitor normal body temperature as well. Totally blocking TRPV1 nerve signals results in elevated body temperature, hyperthermia.
Much of the biological value of proteins is related to their ability to maintain unique molecular shapes. Often a change in a protein's shape changes some of its chemical properties, thereby promoting or discouraging a particular chemical reaction. The above described opening of channel pores in the cell membranes or nerves is one example. We also use this to preserve food by canning. Sufficient heating of the food after packaging in a sealed container changes the shapes of proteins in any microorganisms present. If there is enough shape changes in the proteins which microorganisms needs to survive, the microorganisms die and become part of the food rather than cause the food to spoil. The change of molecular shape does not change the stored energy in the food (measured as Calories) or the contained amino acids and nucleic acids which we digest and reassemble into larger molecules needed for our own bodies. So while the cooking does sterilize the food and somewhat change its color and texture, cooking need not significanly change the nutritional value of the food. (Higher temperatures break the stronger chemical bonds when food is browned or burnt.)
Communicating technical information such as observations and findings is a skill used by scientists but useful for most others. If you need course credit, use your observations in your journal to construct a formal report.
![]()
to next investigation
to Biochemistry menu
to ie-Chemistry menu
to site menu