![]()
The following gets progressively heavier reading. (A lot of chemistry is introduced as we delve deeper into how the nerves detect odors.) You may want to re-read several times slowly, pausing to translate each sentence to your own words, or take some breaks to ponder what is introduced in each paragraph. One might initially wonder why it is so complex.
The challenge is how a collection of chemical molecules
could ever detect and learn about its surrounding environment! The beauty of the system is that it works for organisms both as primitive as single celled yeast and as complex as humans. With minor modifications the same system is used for the cells in multi-cellular animals to coordinate with each other, to detect sound, light, food, smells, pressure and temperature and respond with pleasure, pain, feeding, mating, fear, anger, even self awareness and memory! Such a system is surely worth the effort to understand.
![]()
The ability to detect the odor of molecules is probably the oldest of senses for life on earth. The most primitive single celled organisms living in water have the ability to taste the presence of food around them. While we often distinguish our sense of smell from our sense of taste, the distinction is largely a matter of location rather than fundamental differences. Similar chemical structures and mechanisms are used by all of our senses as well as in other cells types, even those of primitive organisms. This suggesting a history of diverging evolution rather than sensory systems which developed independently.
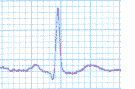 Biochemistry 2 described a mechanism where receptors (sensory portion of nerve cells) could be provoked by external stimuli to initiate electrical signals which carry information to the brain. Most living cells have a small electrical potential (measured as Voltage) across their outer membrane. At the ends of receptors, channels in their outer membrane can be stimulated to open allowing concentrations of ions to flood across the membrane towards lower concentration, discharging that electrical potential. This in turn causes a cascade of neighboring channels to open, carrying the Voltage spike (as graphed at left) along the membrane surface to the distant end of the neuron (nerve cell). The sense of taste uses a similar mechanism. Taste is generated by sensory capsules called taste buds concentrated on the upper surface of the tongue. There chemical reactions rather than mechanical stimulation cause the opening of gateways controlling the channels through the outer cell membranes.
Biochemistry 2 described a mechanism where receptors (sensory portion of nerve cells) could be provoked by external stimuli to initiate electrical signals which carry information to the brain. Most living cells have a small electrical potential (measured as Voltage) across their outer membrane. At the ends of receptors, channels in their outer membrane can be stimulated to open allowing concentrations of ions to flood across the membrane towards lower concentration, discharging that electrical potential. This in turn causes a cascade of neighboring channels to open, carrying the Voltage spike (as graphed at left) along the membrane surface to the distant end of the neuron (nerve cell). The sense of taste uses a similar mechanism. Taste is generated by sensory capsules called taste buds concentrated on the upper surface of the tongue. There chemical reactions rather than mechanical stimulation cause the opening of gateways controlling the channels through the outer cell membranes.
Organic molecules have a backbone chain of Carbon atoms (symbol C) attached together by covalent bonds (one or more shared pairs of electrons). Nearly all the small organic molecules which we smell have additional clusters of atoms which dominate the chemical properties of the molecule. Generally these functional groups, as they are called, contain atoms such as Oxygen (O), Nitrogen (N), and occasionally Phosphorus (P) or Sulfur (S) which all attract electrons more strongly than Carbon. Chemists classify organic molecules by whichever functional group predominates a molecule's chemistry. The current classification system systematically labels by adding a suffix on the molecule's name based on the functional group (although a lot of former names continue in common use).
| functional group's name |
atoms in functional cluster |
classification of a molecule containing functional group |
suffix added to name |
example(s) (alternate common name) |
| hydroxyl | -OH | alcohol | -ol | ethanol = (ethyl alcohol) |
| carbonyl (on end of a chain) |
C=O | aldehyde | -al | methanal = (formaldehyde) |
| carbonyl (elsewhere than on a chain end) |
C=O | ketone | -one | 2 propanone = (acetone) |
| carboxyl | COOH | acid | -oic acid | ethanoic acid = (acetic acid) |
| amine | -NH2 | amine | -ine | adenine, guanine |
| phosphate | -PO4 | phosphate | -P | ATP, cyclic AMP |
Proteins are one of four general types of complex (containing multiple functional groups) organic molecules widely used in living organisms. (The other three types are carbohydrates, lipids, and nucleic acids.) Proteins are generally long chain-like molecules composed of relatively small organic molecules linked together. Each of the smaller subunits in the protein is an amino acid, a molecule with both an amine functional group and also a carboxyl group. (The two formulas below, left of the arrow, are examples.) Note every Carbon atom has four chemical bonds. The central Carbon atom between the amine and carboxyl functional groups is usually attached to Hydrogen (H, which attracts electrons about the same as Carbon, so is chemically rather bland and electrically almost invisible) and a side group which provides diversity, either a third functional group, a short Carbon chain, sometimes a ring of Carbon atoms, or a combination. These variations are here represented by the R and R'. Due to the variations in this R group, there are about 20 different common amino acids and additional rarer varieties. In the equation below, the carboxyl group of the first amino acid reacts with the amine in the second amino acid, forming a water molecule and a small protein composed of the two amino acids joined together by a (highlighted blue) peptide bond. The remaining free amine group on the left end and the free carboxyl group on the right end could both react further forming additional peptide bonds to other amino acids, forming a longer protein chain. Using such peptide linkages, proteins often containing hundreds of amino acids. The particular sequence of the various possible side groups (the Rs) helps determine the properties and function of each protein.
One type of taste receptor contains gated channels made of proteins which are triggered to open by the presence of excess Hydrogen ions, H+. Our brains interpret electrical transmissions from these receptors as the sensation we call sour.
Another type of gated protein channel is triggered by the presence of Chloride ions, Cl-, giving the taste sensation of salty.
Other tastes involve protein gateways which open in responds to particular chemical reactions which occur when a combination of functional groups align with one or more receptor regions on protein gateways. Sweetness is often connected to aldehydes and ketones. Reaction with at least two different sweetness receptors is need to produce the sensation of sweetness. Compounds bind with varying bond strength to the two receptors resulting in perception of different amounts of sweetness. Many of the simplest carbohydrates are sweet. But the tiny protein called aspartame (containing only 2 common amino acids, aspartic acid and phenylalanine) is also extremely sweet. (As a result it carries little nutrition energy as measured by Calories and is therefore used in diet beverages.)
Bases (alkalis) which contain the hydroxide ion, OH– or at least consume Hydrogen ions, H+, are corrosive to tissue and taste bitter, discouraging consumption. Humans eat few substances which taste bitter. Coffee, unsweetened chocolate, uncured olives, dandelion greens and quinine found in tonic water are only slightly basic. There are genetically determined variations in the bitter sensing receptor such that some people find the synthetic substances, phenylthiocarbamide (PTC) and 6-n-propylthiouracil (PROP) to taste very bitter while others find them completely tasteless.
Umami has been suggested as a fifth distinct taste, exemplified by the common solo amino acid glutamate sold as a salt (mono-sodium glutamate), MSG. Umami is considered a fundamental taste in Chinese and Japanese cooking, but is prominent in parmesan and roquefort cheese as well. Umami taste is also provided by the nucleic acids 5'-inosine monophosphate (IMP) and 5'-guanosine monophosphate (GMP) which are naturally present in many protein-rich foods.
Some foods, such as unripe fruits, contain tannins or calcium oxalate that cause an astringent sensation. Tea, rhubarb, grapes and unripe bananas provide this sensation. Recent research suggests an additional taste receptor reacting to fatty acids.
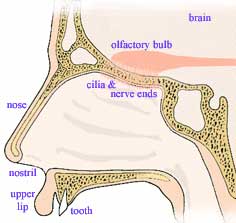 When we inhale a breath of air, it passes first upward through a cavity just below the brain before passing down past the back of the mouth, through the neck's wind pipe, finally branching into the two lungs. The upward initial direction serves the function of normally precluding inhaling objects (think rain and sand) large enough to fall downward. Nose hairs help block smaller dust as do sticky, mucus-covered hairs further down the passageway which constantly sweep their trappings upward. The first get cleaned when we
When we inhale a breath of air, it passes first upward through a cavity just below the brain before passing down past the back of the mouth, through the neck's wind pipe, finally branching into the two lungs. The upward initial direction serves the function of normally precluding inhaling objects (think rain and sand) large enough to fall downward. Nose hairs help block smaller dust as do sticky, mucus-covered hairs further down the passageway which constantly sweep their trappings upward. The first get cleaned when we blow our nose
and the latter generally self clean, sweeping catchings to the esophagus where they are subconsciously swallowed and eventually digested or discharged.
Upon exhaling, air passes upwards past the back of our throats, bringing along samples of small volatile food molecules, released by chewing and vaporized by the warmth of our mouth. At the top of the nasal passage, both inhaled and exhaled air pass a postage-stamp-sized patch (5 cm2) of sensory nerve endings hanging from the bottom of the brain like a miniature upside-down shag rug called the olfactory epithelium. Located here between our eyes are millions of odor detecting nerves, mucus generating cells and a basal layer of stem cells. Each odor detecting nerve has 8 to 20 microscopic-yarn-like cilia. Each such receptor nerve lives about two months before a replacement nerve is grown from the stem cell layer. This rug like tissue is kept damp with mucus which helps adsorb molecules from the passing air. On a still smaller molecular scale, detector proteins, threaded back and forth across the outer membrane of the nerve cilia, react with the small volatile molecules triggering pore openings, initiating the nerve signals sent to the brain. The mechanism by which odor is detected and the information sent on to the brain and processed has been untangled by teams led by Linda B. Buck and Richard Axel resulting in their receiving the 2004 Nobel Prize in Physiology or Medicine.
In general, functional groups on the small molecules carried in the passing air temporarily react with a selection of detector proteins sticking out of the surface membrane of the nerve cilia hanging from the olfactory epithelium. Humans can distinguish between 10,000 and 100,000 different smells. This is partly due to a large number of specialized detector proteins (called receptor proteins), each type on its own specialized olfactory nerve, and partly due to the pattern of signals from multiple nerves. In both mice and humans, no less than 3% of the genes provide code for the manufacture of receptor proteins. Mice have as many as 1000 different detector proteins while human genes only produce about 350 distinct detector proteins.
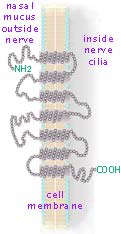 Like most chains, these proteins (with thousands of small amino acids hooked together by the peptide bonds) are supposedly free to rotate into different shapes. That is, each single bond resulting from the covalent sharing of a pair of electrons does not restrict free rotation. However when a particular protein with a specified sequence of amino acids rotates, some of the protruding side functional groups (recall the R and R' in the reaction above) encounter functional groups on neighboring molecules (or elsewhere along their own chain). These form weak bonds which restrain further motion. The result is that most proteins actually maintain unique coiled and folded shapes with only limited flexibility. In the case of the receptor proteins embedded in a nerve's outer membrane, their shape is further restrained by attractions to that cell membrane. These proteins have 7 coiled sections which experience (non-polar) attractions to the double layer of lipids which compose cell membranes. So each receptor protein laces through a nerve cilia's surface membrane 7 times (as shown at right→. Each little gray dot represents an amino acid. The end functional groups are exagerated in size to be readable.) There are millions of detector cells in the olfactory epithelium. The 8 to 20 cilia dangling from any particular detection nerve cell have only a single type (of the various 350 types in humans) of receptor protein entwined in its cell membrane. When an odor molecule's specific functional group or spacial arrangement of several functional groups aligns and temporarily docks with a matching exposed segment of detector protein, the detector protein changes shape setting off a chain of chemical reactions starting the nerve's electrical signal.
Like most chains, these proteins (with thousands of small amino acids hooked together by the peptide bonds) are supposedly free to rotate into different shapes. That is, each single bond resulting from the covalent sharing of a pair of electrons does not restrict free rotation. However when a particular protein with a specified sequence of amino acids rotates, some of the protruding side functional groups (recall the R and R' in the reaction above) encounter functional groups on neighboring molecules (or elsewhere along their own chain). These form weak bonds which restrain further motion. The result is that most proteins actually maintain unique coiled and folded shapes with only limited flexibility. In the case of the receptor proteins embedded in a nerve's outer membrane, their shape is further restrained by attractions to that cell membrane. These proteins have 7 coiled sections which experience (non-polar) attractions to the double layer of lipids which compose cell membranes. So each receptor protein laces through a nerve cilia's surface membrane 7 times (as shown at right→. Each little gray dot represents an amino acid. The end functional groups are exagerated in size to be readable.) There are millions of detector cells in the olfactory epithelium. The 8 to 20 cilia dangling from any particular detection nerve cell have only a single type (of the various 350 types in humans) of receptor protein entwined in its cell membrane. When an odor molecule's specific functional group or spacial arrangement of several functional groups aligns and temporarily docks with a matching exposed segment of detector protein, the detector protein changes shape setting off a chain of chemical reactions starting the nerve's electrical signal.
All the proteins which form the channels through cell membranes and serve as gates and receptors are of a type of protein now labelled guanosine nucleotide binding proteins, or simply G-proteins. Alfred Gilman and Martin Rodbell discovered and investigated these G-proteins and consequently were awarded the Nobel Prize in Physiology or Medicine in 1994 for their achievements. It turned out that these proteins do not function as single molecules, but work as parts of protein clusters with mass of about 1500 kDa. And often neighboring clusters provide supporting functions for each other. An example of that is described below.
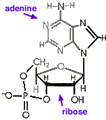
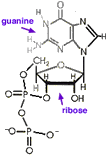 Like most processes in life, an energy source is required for receptors to detect tastes and odors. As with other cell processes, energy is commonly transported by two nucleic acids, adenine and guanine. These are the same two double-ring nucleic acids also used in DNA and RNA, often abbreviated simply A and G. Each is attached to ribose, an Oxygen rich carbohydrate, just as they are in Ribonucleic acid, RNA. But unlike in RNA where nucleotides are combined into long chains fastened together by intervening phosphate functional groups, when used to transport energy these nucleic acids are not chained together, but instead each is coupled through ribose to three phosphates making molecules called adenosine triphosphate, ATP, and guanosine triphosphate, GTP. Energy is provided when these molecules release phosphate forming 3'5'adenosine monophosphate (also called cyclic-AMP, ←structural formula above left. Note that to simplify, Cs for Carbon at ring corners are not labelled), and similar guanosine diphosphate (cyclic-GDP, above right→). The G-proteins get their name because they attach to guanosine.
Like most processes in life, an energy source is required for receptors to detect tastes and odors. As with other cell processes, energy is commonly transported by two nucleic acids, adenine and guanine. These are the same two double-ring nucleic acids also used in DNA and RNA, often abbreviated simply A and G. Each is attached to ribose, an Oxygen rich carbohydrate, just as they are in Ribonucleic acid, RNA. But unlike in RNA where nucleotides are combined into long chains fastened together by intervening phosphate functional groups, when used to transport energy these nucleic acids are not chained together, but instead each is coupled through ribose to three phosphates making molecules called adenosine triphosphate, ATP, and guanosine triphosphate, GTP. Energy is provided when these molecules release phosphate forming 3'5'adenosine monophosphate (also called cyclic-AMP, ←structural formula above left. Note that to simplify, Cs for Carbon at ring corners are not labelled), and similar guanosine diphosphate (cyclic-GDP, above right→). The G-proteins get their name because they attach to guanosine.
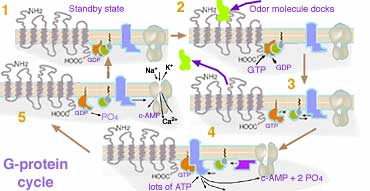
In the standby state (1) of the (here diagrammed gray) odor detector molecule, G-protein, a segment inside the cilia is bound to one (here orange) GDP molecule. When the (yellow-green) odor molecule docks with the outside sections of the G-protein (2), the GDP is released, and a nearby energy rich version, GTP, immediately attaches to the vacated site, causing the G-protein cluster to change shape setting off a cascade of other reactions much like many timing devices. The shape change causes the odor molecule to be ejected (3) so it might move on to trigger other suitable neighbor detector molecules. Within a second or so the G-protein hydrolyzes the GTP to GDP, turning off the other reactions and using the released energy to soon reset itself back to the standby mode. By acting like a timer switch, the G-protein provides a mechanism for great amplification such that the detection of a single odor molecule subsequently produces a large number of molecules before the standby state is restored. While the G-protein is in its last stages of activity (4), it catalyzes the decomposition of many ATP molecules to adenosine monophosphate. In this molecule the lone remaining phosphate attaches to both positions labeled 3' and 5' forming a 4th (cyclic) ring. (This molecule was discovered and called cyclic-AMP in 1957 by Earl Sutherland who was awarded the Nobel Prize in 1971 for the discovery of its role with adrenalin. Cyclic-AMP acts as an intracellular messenger that mediates the actions of hormones and other outside molecules on many aspects of cellular metabolism, growth, and differentiation. Further investigation about its mechanism led to Gilman and Rodbell's discovery of G-proteins.) But in this mechanism for detecting odor, the large number of cyclic-AMP formed cause nearby cyclic-nucleotide gated (CNG) channels in the cilia membrane to open allowing Na+ and Ca2+ ions to flood in and K+ to flow out (5). This rapidly neutralizes then reverses the electrical potential on the membrane surface nearby. The electrical discharge rolls like an avalanche also triggering Voltage activated gates to open, spreading the discharge along the nerve as the Voltage spike moves like a signal wave the length of the nerve fiber.
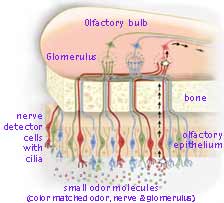
The electrical discharge set off along a nerve's surface, rises through a tiny bone canal to ganglia cells just above in the front portion of the brain known as the olfactory bulb. (For simplicity, only 3 of the 350 types are shown color coded in the diagram→.) Here glomeruli integrate signals from matching detector nerve cells forming a stronger signal. Each glomerulus receives input from several thousand detector nerve cells but only from those which contain the same identical kind of detector G-protein. These glomeruli are long-lived nerve cells which provide for consistent processing over time even though the receptor cells die and are replaced by new nerves grown from stem cells. From a glomerulus in the olfactory bulb, a nerve signal is sent backwards into various olfactory cortex regions of the brain (with both brain halves mirroring each process).
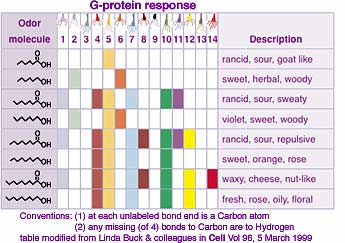
Each particular odor message is mapped to specific brain locations but these overlap somewhat with locations for other odors, apparently facilitating coincidence analysis. The odor detected depends on the pattern of electrical discharges from the collection of nerves rather than each nerve corresponding to a particular odor. (See examples at left.) Lower concentration of odor molecules results in detection by fewer cilia, and consequentially a different pattern, sometimes giving the sensation of a completely different odor. The odor signal is then forwarded to other parts of the brain for odor discrimination, emotional and physiological effects, and memory.
In addition to the relatively small odor molecules, the olfactory system also detects pheromones. The first of these substances was first isolated in the 1950s and shown to be a chemical communication system used by insects. While thousands of insect pheromones have been chemically identified, it is now known that pheromones are released by many kinds of animals and act on members of the same species, stimulating hormonal changes or instinctive behaviors, such as mothering, mating, aggression and fear. Pheromones are detected primarily in the vomeronasal organ, a separate olfactory structure in the nasal septum. While the scent of animals in heat was known long before, the chemistry of pheromones in higher animals and humans is still poorly understood.
Experiments have show that our brains receive information about odors and can respond even though we have no conscious awareness.
With well understood concepts of science, a teacher can often select experiments which will help a student understand that concept. While that may still be the intent with less well understood concepts, a more crucial function might be for students to try to develop their own investigations to verify if a concept is correct or needs revision or extension. That is, students can themselves become part of the science process, checking, testing, and perhaps extending human understanding. In such areas where our understanding has been only recently achieved, where much speculation exists, there remains good possibility of error and need for revision based on investigations not previously envisioned or conducted. Understanding of our senses is such an area ripe for further progress!
I hear a 200 pound bear was killed 1/2 block away from my back door last night... [It was] One of those things you never know: I was doing a puzzle and had fallen asleep in my recliner. I woke up after midnight (when I now know it happened). I went to put the dog out one last time before we went up to bed. I usual flip on the yard light, glance out, and let her out, but last night I went out and stood in my back yard and looked around for a while before letting the dog out. I immediately felt something was not right, BUT felt no fear or worry. None of the pets inside were at all upset [although they typically sense anything] unusual... before I do. I still reacted to something not right, all on a subconscious level. As quiet as the night was, I heard NO unusual sounds... I stood out there for many minutes before I let her out. I stayed out there until she was ready to come back in. In 16 years, this was the first time I had done this. I even wondered WHAT/WHY I was doing as I was standing out there.Perhaps this was a subconscious detection of pheromone from the nearby bear? That possibility seems more plausible than hearing any bear noise while inside the house. (The bear was not shot but after wandering the neighborhood for hours, later died of unknown cause while facing wild life officers trying to arrange capture for relocation to an uninhabited area.) While experimenting with a wild bear is likely more dangerous than necessary, this incident might provide enough clue to allow designing a pioneering experiment to investigate the possibility of subconscious human detection of pheromones in such situations.
Communicating technical information such as observations and findings is a skill used by scientists but useful for most others. If you need course credit, use your observations in your journal to construct a formal report.
![]()
to next investigation
to Biochemistry menu
to ie-Chemistry menu
to site menu