Since antiquity, motion has been looked upon as the index of life. The organ of motion is muscle. (Andrew G. Szent-Györgyi)
![]()
Since antiquity, motion has been looked upon as the index of life. The organ of motion is muscle. (Andrew G. Szent-Györgyi)
![]()
 In 1864 a viscous protein was extracted from muscle with concentrated salt solution by W. Kühne who called it myosin and considered it responsible for the rigor state of muscle. In 1842 the great German physiologist, histologist and cytologist Theodor Schwann (b1810, d1882) described muscle cells vaguely as chemical-mechanical devices, meaning that they convert chemical energy into mechanical work. Other than food was the primary fuel, very little was understood about the chemical process until the German chemist Karl Lohmann (b1898, d1978) discovered ATP in 1929. In 1934 Lohmann reported that ATP is a molecule essential for muscle contraction and likely the energy source for contraction. In 1939 the Russian husband and wife team V.A. Engelhardt and M.N. Lyubimova established that the myosin actually uses the ATP. Engelhardt and Lyubimova created the term mechanochemistry to embody the concept that the protein myosin which carries out the work also liberates the energy necessary for the work. By the early 1940's Andrew Szent-Györgyi (←at left) had established that the myosin used by previous investigators consisted of two proteins. F.B. Straub isolated and purified actin in 1942. Szent-Györgyi purified myosin as paracrystals in 1943 and chose to retained the name myosin. Szent-Györgyi created contraction in a test tube by placing synthetic actin and myosin filaments together in the presence of ATP. Myosin could not contract alone, therefore muscle contraction must involve interaction of actin, myosin and ATP.
In 1864 a viscous protein was extracted from muscle with concentrated salt solution by W. Kühne who called it myosin and considered it responsible for the rigor state of muscle. In 1842 the great German physiologist, histologist and cytologist Theodor Schwann (b1810, d1882) described muscle cells vaguely as chemical-mechanical devices, meaning that they convert chemical energy into mechanical work. Other than food was the primary fuel, very little was understood about the chemical process until the German chemist Karl Lohmann (b1898, d1978) discovered ATP in 1929. In 1934 Lohmann reported that ATP is a molecule essential for muscle contraction and likely the energy source for contraction. In 1939 the Russian husband and wife team V.A. Engelhardt and M.N. Lyubimova established that the myosin actually uses the ATP. Engelhardt and Lyubimova created the term mechanochemistry to embody the concept that the protein myosin which carries out the work also liberates the energy necessary for the work. By the early 1940's Andrew Szent-Györgyi (←at left) had established that the myosin used by previous investigators consisted of two proteins. F.B. Straub isolated and purified actin in 1942. Szent-Györgyi purified myosin as paracrystals in 1943 and chose to retained the name myosin. Szent-Györgyi created contraction in a test tube by placing synthetic actin and myosin filaments together in the presence of ATP. Myosin could not contract alone, therefore muscle contraction must involve interaction of actin, myosin and ATP.
 Structural studies by Ralph Niedergerke and Andrew F. Huxley (b 1917, near right→, who won a Nobel Prize for earlier work on nerves) and by Jean Hanson (b1919, d1973, center) and Hugh E. Huxley (far right →) using x-ray diffraction, light microscopy and electron microscopy provided images of an ordered matrix of two sets of inter-digitating fibers. These two sets of fibers, called the thick and thin filaments, are predominantly myosin and actin respectively. In adjoining papers published in 1954, both groups proposed the sliding filament theory that during muscle contraction the actin and myosin filaments slide past each other so that they overlap, contracting the length of the muscle, but without any change in filament lengths.
Structural studies by Ralph Niedergerke and Andrew F. Huxley (b 1917, near right→, who won a Nobel Prize for earlier work on nerves) and by Jean Hanson (b1919, d1973, center) and Hugh E. Huxley (far right →) using x-ray diffraction, light microscopy and electron microscopy provided images of an ordered matrix of two sets of inter-digitating fibers. These two sets of fibers, called the thick and thin filaments, are predominantly myosin and actin respectively. In adjoining papers published in 1954, both groups proposed the sliding filament theory that during muscle contraction the actin and myosin filaments slide past each other so that they overlap, contracting the length of the muscle, but without any change in filament lengths.
In 1957 Hugh Huxley developed more detailed images showing cross-bridges between the actin and myosin. The important electron microscope studies of H.S. Slayter and S.Lowey in 1967 established that long myosin molecule ended in two globules (heads). (The first visual impressions of myosin was of a long-tailed, snake-like polymer with a raised double heads. Later the structure looked more like the cross-bracing of a railroad trestle or perhaps its swinging cross bridge. Perhaps a better comparison might be a many legged caterpillar with big feet.) The following year he proposed that because only 15-25% of these crossbridges are in contact with the actin at any instant, these contacts repeatedly knocked into the actin pushing it along. Removing ATP caused the heads
(or feet
) to stay attached, placing the muscle in rigor (as happens in rigor mortis after death). In 1965 Ken Holmes found that crossbridges increased at right angles in rigor, and dropped loose when ATP was added. This provided the first direct evidence that crossbridges (legs
) actually move. The cycle currently believed accurate was proposed by R.W. Lymn and E.W. Taylor in 1971. In 1986 Roger Cooke based on evidence that less mass moved than expected suggest that rather than the whole cross-bridge moving, instead a part of the tail of the molecule acted as a lever-arm ( or leg
). Wolfgang Kabsch determined the crystal structure of the actin in 1990. In 1994 Ivan Rayment showed different isoforms of myosin with the tail region rotated about the motor domain by about 35°. Procedure to crystallize actin and myosin now allows for a search for the changes in molecular structures to determine how the energy is utilized to produce the contraction force.
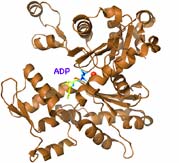
![]() Muscles contain 3 types of protein fibers: microfilaments, microtubules, and intermediate filaments. Microfilaments are polymers composed of globular unit monomers called actin. All actin from algae to humans is chemically nearly identical. Each actin monomer contains an ATP ( an adenine nucleotide bonded to three phosphate groups) in the center of a a coiled and twisted protein which roughly takes the shape of an open mouth. When these monomers polymerize, one phosphate is cleaved off leaving ADP (adenine diphosphate). The mouth shape closes (shown at left; click to enlarge) as if biting hold of the previous monomer. A long chain is formed. Two of these actin polymer chains twine together to form a microfilament (diagram above right).
Muscles contain 3 types of protein fibers: microfilaments, microtubules, and intermediate filaments. Microfilaments are polymers composed of globular unit monomers called actin. All actin from algae to humans is chemically nearly identical. Each actin monomer contains an ATP ( an adenine nucleotide bonded to three phosphate groups) in the center of a a coiled and twisted protein which roughly takes the shape of an open mouth. When these monomers polymerize, one phosphate is cleaved off leaving ADP (adenine diphosphate). The mouth shape closes (shown at left; click to enlarge) as if biting hold of the previous monomer. A long chain is formed. Two of these actin polymer chains twine together to form a microfilament (diagram above right).
Microfilaments are present in other types of cells as well. They are assembled and disassembled, causing a cell's shape to change and sometimes encourage the cell to move in one direction as in the motion of white blood cells. Microtubules are polymers composed of two monomers, α and β tubulin. These monomers contain the nucleotide guanine instead of adenine. They are involved in many cellular processes including mitosis, cytokinesis, and vesicular transport. Intermediate filaments contain other polymers such as keratin and don't contain nucleotides in their monomers. They compose structures inside cells but are more familiar in external forms such as hair, nails, horns and scales. But the primary fibers involved in locomotion and modification of surroundings are the microfilaments which function as described below.
walking feetwhich reach out to either side and walk along the movable skateboard-like actin. (A pair of legs & feet of Homo Sapien myosin is shown right. Other kinds vary widely. The protein structure of a foot is shown below left; click to enlarge)
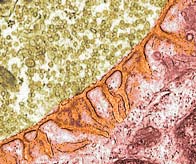 A neuron carries a signal along the outer membrane of its long axon to the nerve terminal adjacent to a muscle cell. The neurotransmitter acetylcholine is pre-packaged in balloon-like membranes inside the terminal (colorized in microscope image tan). As the signal travels along the membrane, gateways are triggered to open allowing Calcium ions to diffuse into the neuron. At the terminal, these Calcium ions cause the balloon membranes to merge with the neuron's outer membrane, releasing the acetylcholine contents into the narrow gap of the synapse between the neuron and the muscle cell (colorized in microscope images: synapse orange, muscle red ). The highly folded muscle cell membrane has a large surface area to optimize available receptors for docking of the acetylcholine. Upon the docking, Sodium channels in the muscle membrane are opened discharging the electrical potential across the membrane (measured as a Voltage spike). This change in electrical potential sweeps across the muscle's outer membrane but also travels inward along T-tubules. The acetylcholine is subsequently degraded by an enzyme to prepare for another signal.
A neuron carries a signal along the outer membrane of its long axon to the nerve terminal adjacent to a muscle cell. The neurotransmitter acetylcholine is pre-packaged in balloon-like membranes inside the terminal (colorized in microscope image tan). As the signal travels along the membrane, gateways are triggered to open allowing Calcium ions to diffuse into the neuron. At the terminal, these Calcium ions cause the balloon membranes to merge with the neuron's outer membrane, releasing the acetylcholine contents into the narrow gap of the synapse between the neuron and the muscle cell (colorized in microscope images: synapse orange, muscle red ). The highly folded muscle cell membrane has a large surface area to optimize available receptors for docking of the acetylcholine. Upon the docking, Sodium channels in the muscle membrane are opened discharging the electrical potential across the membrane (measured as a Voltage spike). This change in electrical potential sweeps across the muscle's outer membrane but also travels inward along T-tubules. The acetylcholine is subsequently degraded by an enzyme to prepare for another signal.
The muscle contraction is started by the sweeping change in electrical potential flowing into the muscle cell along the T-tubules. This Voltage spike triggers membrane gates to open allowing Calcium ions stored around the muscle fibers to flood in. The increase in Ca2+ concentration from approximately 10-7 to 10-5M, allows bonding of the myosin feet (heads) to the adjacent actin giving traction to the myosin footwork. Two accessory proteins, tropomyosin and troponin, bound to the actin filaments assist in establishing traction. Tropomyosin is a fibrous protein that binds lengthwise along the groove of actin filaments. In striated muscle, each tropomyosin is bound to troponin, which is a complex of three smaller protein: troponin C (Ca2+-binding), troponin I (inhibitory), and troponin T (tropomyosin-binding). At low Ca2+ concentrations, the complex of the troponins with tropomyosin blocks the myosin from getting a footing on the actin. At high concentrations, Ca2+ bind to troponin C shifting the position of the complex out of the way, permitting a foothold. Once a footing is established, the myosin can dock an ATP molecule and receive the energy necessary to cycle of contraction process. Mitochondria occupy the cell volume surrounding the microfilaments and as a byproduct of their own activity, generate extra ATP which myosin use to power muscle contraction.
Calcium ions are eventually pumped out of the muscle fibers, preventing the myosin feet from their footholds on the actin microfilaments. This relaxes the muscle. But without the bonding to the actin, the myosin can no longer refuel with ATP, thus the walking cycle stops unless new nerve signals are received.
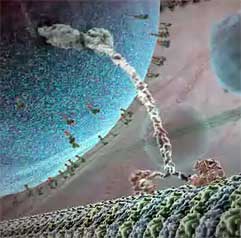 Other myosin and the similar kinesin and dynein also walk but often over much longer distances. Biologists have long witnessed microscopic images of cell division where chromosomes were pulled apart to form two separate nuclei. Mitochondria are also tugged to locations where ATP is needed. And in the long neurons, capsules containing neurotransmitters must be transported from manufacture location to distant synapse. Transport which would take years by diffusion is accomplished in minutes or less by protein motors which walk along microtubules composed of α and β tubulin (very similar to actin), pulling along much larger packages. Most large (1,500 kDa) dyneins tend to walk towards the center moving vesicles packaged near the outer cellular membrane, while most much smaller (150 kDa) kinesins pull packages outward for export. While myosin typically takes steps averaging 36 nm along relatively thin strands of actin, the dynein and kinesin take smaller 8 nm steps along microtubules three times the diameter of actin. Most of these walking proteins are dimers with two legs which twist back and forth around an α-helical coil (as shown in frame to right, from the inner life of the Cell. Click to link to movie excerpt.) At the other tail (here upper) end of the coil they attach to cargo needing transport. At the end of each leg is a fat foot-like segment which attach a footing to their walkway fiber, then upon bonding with an ATP, steps onward to get a new footing further along the fiber. Except for dynein, each type of walking motor only walks one direction along their fiber. Viruses and some infectious bacteria, including HIV, use a cell's dyneins to transport their DNA (or RNA) to a cell's nucleus for reproduction.
Other myosin and the similar kinesin and dynein also walk but often over much longer distances. Biologists have long witnessed microscopic images of cell division where chromosomes were pulled apart to form two separate nuclei. Mitochondria are also tugged to locations where ATP is needed. And in the long neurons, capsules containing neurotransmitters must be transported from manufacture location to distant synapse. Transport which would take years by diffusion is accomplished in minutes or less by protein motors which walk along microtubules composed of α and β tubulin (very similar to actin), pulling along much larger packages. Most large (1,500 kDa) dyneins tend to walk towards the center moving vesicles packaged near the outer cellular membrane, while most much smaller (150 kDa) kinesins pull packages outward for export. While myosin typically takes steps averaging 36 nm along relatively thin strands of actin, the dynein and kinesin take smaller 8 nm steps along microtubules three times the diameter of actin. Most of these walking proteins are dimers with two legs which twist back and forth around an α-helical coil (as shown in frame to right, from the inner life of the Cell. Click to link to movie excerpt.) At the other tail (here upper) end of the coil they attach to cargo needing transport. At the end of each leg is a fat foot-like segment which attach a footing to their walkway fiber, then upon bonding with an ATP, steps onward to get a new footing further along the fiber. Except for dynein, each type of walking motor only walks one direction along their fiber. Viruses and some infectious bacteria, including HIV, use a cell's dyneins to transport their DNA (or RNA) to a cell's nucleus for reproduction.
More primitive types of cells may use the spinning ATPase protein complexes to drive flagella which essential propel the cell via the paddling technique known as sculling.
to be developed
Communicating technical information such as observations and findings is a skill used by scientists but useful for most others. If you need course credit, use your observations in your journal to construct a formal report.
 Andrew G. Szent-Györgyi, The Early History of the Biochemistry of Muscle Contraction, Brandeis University, 2004
Andrew G. Szent-Györgyi, The Early History of the Biochemistry of Muscle Contraction, Brandeis University, 2004![]()
to next investigation
to Biochemistry menu
to e-Chemistry menu
to site menu