![]()
![]()
Reminder: It is appropriate to occasionally think about what we hope to achieve and what we have learned already. This page is offered to assist with evaluating that progress.
Part I:
As in experiment 4.1, a colored solid (such as koolaid) was dissolved in water in a container perhaps as shown at right.
If more water is added, what will happen to the color?
If instead, more solid is added, what will happen to the color?
When the amount of substance that dissolves in a solvent reaches its limit, the solution is said to be...
 5.00 g of table salt (sodium chloride, formula NaCl) is mixed in 5 cm3 of room temperature water but not all the solid dissolves. All of the liquid is next carefully poured into an evaporating dish. This separation from the solid is called
5.00 g of table salt (sodium chloride, formula NaCl) is mixed in 5 cm3 of room temperature water but not all the solid dissolves. All of the liquid is next carefully poured into an evaporating dish. This separation from the solid is called
The dish was heated until all the water boiled away. After cooling, the dish contained 1.62 g of solid. What was the solubility of NaCl (in g/100cm3 of water)?
Approximately 34g of oxygen gas will dissolve in 1000mL of 20°C water. What is the solubility of oxygen (in g/100cm3 of water)?
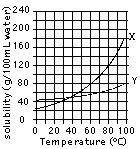 A graph to the right was made by dissolving two different solids in water at various temperatures.
A graph to the right was made by dissolving two different solids in water at various temperatures.
According to the graph, how much of substance Y would dissolve in 100 cm3 of water at 20°C?
What is the lowest temperature that 9Og of substance X will dissolve in 100 cm3 of water?
100 cm3 of water at 20°C is saturated with substance X. If it is warmed to 100°C, how much MORE X can he dissolved?
If a saturated solution of either X or Y is cooled, some of the solid will settle out of the solution. In this process the solid that “settles out” is said to...
Do most solids dissolve better when water is hot or cold?
Which substance(s) tested in experiment 4.4 dissolved in the alcohol but NOT water?
Which substance(s) tested in experiment 4.4 dissolved in BOTH the alcohol (methanol) AND water?
The alcohol methanol could be made by heating which substance?
| Temperature (°C) |
Nitrogen | Carbon dioxide |
Sulfur dioxide |
Hydrogen | Ammonia |
| 0 | 0.0024 | 0.34 | 23 | 82 | 90 |
| 20 | 0.0019 | 0.17 | 11 | 72 | 53 |
| 40 | 0.0014 | 0.10 | 5.5 | 63 | 32 |
| 60 | 0.0011 | 0.07 | 3.3 | 56 | 14 |
Based on the table of gas solubilities at different temperatures, how much ammonia can be stored in room one Liter of room temperature water?
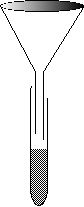 A solution called limewater can be made by saturating lime, Ca(OH)2, in water then removed any undissolved solid. When filtered, where would the residue collect?
A solution called limewater can be made by saturating lime, Ca(OH)2, in water then removed any undissolved solid. When filtered, where would the residue collect?
Where did the filtrate collect?
How do you prepare filter paper so it is ready for filtering?
If you gently heat and evaporate the limewater, what would you find remaining in the evaporating dish?
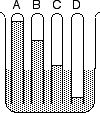 Four tubes of various gases were uncorked in a beaker of water. After a minute they looked like the diagram -->. Which gas is most soluble in water?
Four tubes of various gases were uncorked in a beaker of water. After a minute they looked like the diagram -->. Which gas is most soluble in water?
If one of these gases was PURE air, in which tube was it?
In the 1930s the German Zeppelin Company provided high speed transportation across the Atlantic Ocean in airships such as the LZ-129, Hindenburg, containing Hydrogen. The density of hydrogen gas at atmospheric pressure must beA glowing splint quickly goes out when inserted into a gas that is more dense than air.
What is the name for this gas?
A small explosion occurs when a burning splint is inserted into a gas that's less dense than air. Based on the gases you have seen tested, what is the name of this gas?
If limewater turns cloudy when a gas bubbles through it, this gas is probably...
If a splint test shows that a gas is still in a tube open downward, but not in an open upright tube, the density of that gas compared to air is...
On earth, the gas that turns limewater cloudy is made bySome solids dissolve in ground water. These sometimes effect the taste of tap water, but they also leave stains in sinks, tubs, shower stalls, and other locations where water is allowed to evaporate. When such solids are dissolved in water the water is called...
What gases are primarily responsible for causing acid rain?
Acid rain can be harmful in our drinking water becauseThere are two kinds of felt-tip pens. Some are labeled permanent
and some are labeled washable.
What does the label tell you about the solubility in water of the dyes in the two inks?
When it rains, water soluble gases are removed from the air. Would this happen better on hot days or cold? EXPLAIN your reasoning: