Physical Science
Evaluate Yourself and Improve
Chapter 1
So far we haven't studied Einstein's Special Relativity, what science can determine about the origins of the universe, cloning life by duplicating DNA, or any of the topics that are most interesting to you.
- If you want to fly a state-of-the-art airplane you can't just jump without training into the cockpit expecting to be a proficient pilot. Similarly becoming a skilled physician requires years of training. Even star athletes require years of tedious practice. So it should be no surprise that science too requires some tedious work.
- Many of the greatest scientists have suggested that the best way to learn science is to repeat the key mental stages of development that led our culture to its current understanding. By personally making major discoveries in historical order, we add understanding piece by piece. By learning how and why each became part, we gain a deep understanding unattainable by just memorizing current vocabulary and equations.
For example, a surgeon needs to know much more than to ask for a scalpel to remove an inflected appendix. Skilled procedures must be followed. A broad understanding is essential in case something goes wrong, needs to be recognized and some corrective action taken. Likewise if we want to understand Special Relativity (or any other part of modern science), we need to know more than vocabulary and a couple equations
(science tools). We'll need to know the skills of when and how to use the equations. We also needed to understand the circumstances that brought Einstein to propose the equations so we appreciate when they apply and when their use would only cause problems.
- While we have not started with the most interesting topics, we should develop a perspective how these topics lead us to our desired understanding.
No generic course of study such as this can be tailored to fully match each diverse individual's interests. But with some guidance you can do that! It is not too early to think about what you hope to achieve from this study and how what you have learned builds in that direction. Periodically we should recall what we hope to accomplish and evaluate our progress. What at first may seem to be wrong may be well directed progress!
Directions
Use the following to evaluate what you understand and what you need to study further:
Part I:
- Make a list of what you hope this course helps you to understand.
- Review what you've learned from the experiments and problems so far to determine the relevancy of observation and measurements to what you hope to understand.
- Ponder the possibility that incomplete observations and measurement difficulties can lead to erroneous conclusions. For example, how certain are you of the chemical composition of the smoke from a candle? How certain are you that the burning candle emitted heat? In what circumstances are you most confident, and under what circumstances are you less confident?
Part II:
- Which of the following are observations and which are interpretations?
- I hear the bell.
- It will snow tomorrow.
- It snowed yesterday.
- Water is composed of H2O.
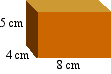
- What would be the volume of a bar 8 cm long, 4 cm wide, and 5 cm thick?
- According to the laboratory standard, under what circumstances is
playing around
in the laboratory acceptable?
- What is a scientist's journal?
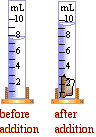 What is the usually name for containers with scales such as the one to the right? What is the vocabulary term for top surface of a liquid?
What is the usually name for containers with scales such as the one to the right? What is the vocabulary term for top surface of a liquid?- Where should the curved top surface be read to properly measure volume?
- What is the volume difference between the smallest divisions marked on these cylinder?
- What is the volume of the liquid in the closest container to the right (Be as precise as possible, remembering units)?
- A rock is added displacing some of the liquid as shown further right. What is the volume of the rock?
- The liquid in the container was water (density 1.0 g/cm3). If methanol alcohol (density 0.78 g/cm3) is used instead, what would be the volume of the rock?
- 50 cm3 of methanol alcohol would be how many mL?
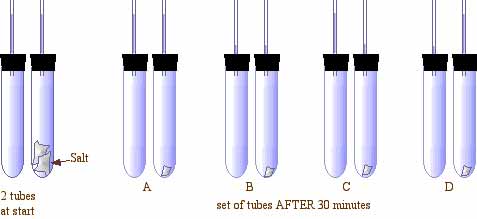
- An experiment shown below was done with two test tubes. One test tube was filled completely with water. A second tube containing some rock salt was also filled with water. Both tubes were capped by rubber stoppers with single holes containing small, open, glass tubes as shown below left. NOTE at the start both were filled equally with water part way up the glass tubes. After 30 minutes, some of the salt dissolves. Which set would best show what the water levels would like after 30 minutes?
- Mass is the
- pull of gravity on matter.
- amount of matter in an object.
- same as weight.
- unit of weight in the metric system.
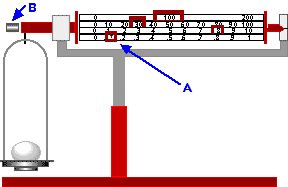
- What is the equipment to the right called?
- What is the part A called?
- What is part B for?
- What is the mass of the egg?
- A balance
- is a kind of scale.
- is used to measure length.
- works with springs.
- compares two masses.
- An object is massed three times: 18.324 g, 18.308 g, 18.342 g. How should the mass be reported?
- Under what conditions is volume a reliable way to measure the quantity of material? Under which conditions does volume change when the no material is actually gained or lost?
![]()
![]()

 What is the usually name for containers with scales such as the one to the right? What is the vocabulary term for top surface of a liquid?
What is the usually name for containers with scales such as the one to the right? What is the vocabulary term for top surface of a liquid?
